Complete Equation Notice For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
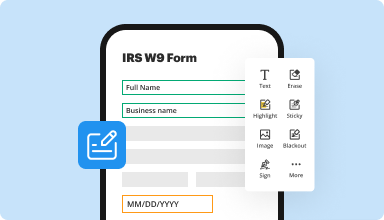
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
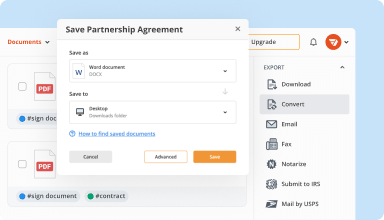
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
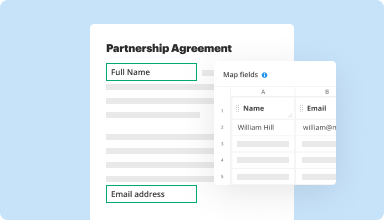
Collect data and approvals
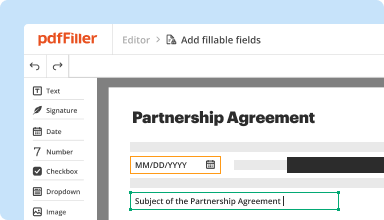
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
We all look for ways to make our day more efficient. If you are tired of printing out documents to only write on, scan and send back this is your answer to cut out the process. Simply type in to the PDF, save and send.
2015-08-27
People want to type into box which needs signed into is one problem another is email comes from PDF filler and a lot of people don't know I'm one sending stuff to be signed
2017-05-31
So convenient and easy
I absolutely love how easy it is to pull a document and fill in information or sign and send back out. This is super simple for the constant paperwork I must complete.
It is difficult sometimes to get the size and format of wording to fit correctly.
2019-08-08
A time saver
A great way to fill, edit and sign PDF documents easily and on the go. Create professional-looking documents even if you are out of the office.
Affordable and efficient.
It includes many customizable templates for different purposes and the Drag and Drop feature makes it easy to use.
Compatible with a variety of formats.
None, really.
It takes some getting used to after using tools such as Adobe, but overall it's pretty intuitive.
2018-03-21
This is my first experience with online documents and I had a difficult time maneuvering through the settings. Would like more information on how to use.
2023-08-17
PDFFiller: A Convenient and User-Friendly PDF Editing Solution
I have been using PDFFiller for several months now and I am impressed with its functionality and ease of use. The software allows me to easily edit and sign PDF documents, saving me a lot of time and hassle. The interface is user-friendly and the features are comprehensive, making it easy to use for people of all skill levels. The mobile app is also very convenient and allows me to access and update my PDFs on the go.
The software is very user-friendly and easy to navigate. It allows me to easily edit and sign PDF documents. The mobile app is also very convenient and allows me to access and update my PDFs on the go.
I did not encounter any major cons while using the software, however, I would like to see more customization options for the templates.
2023-01-16
What do you like best?
It is much easier to use than Adobe Acrobat. Much more intuitive functions and file management. It has saved me a ton of time with the cloud storage of documents. I have used effectively for construction related documents.
What do you dislike?
Very rarely I have needed to use another platform because some municipalities require it but 98% of what I need to do is supported.
What problems are you solving with the product? What benefits have you realized?
Remote completion of forms and extracting text from PDF documents mostly.
2021-02-16
Great customer service staff readily…
Great customer service staff readily available, they don't waste anytime contacting you or getting you the support you need. Highly recommend.
2021-02-03
Very satisfying!!! It has been a very helpful tool to modify and to fill up forms related to my work. The only thing I regret is not have been subscribed earlier.
2020-05-10
Complete Equation Notice Feature
The Complete Equation Notice feature simplifies your experience by providing clear insights into your data and decisions. This feature equips you with the tools needed to make informed choices effortlessly.
Key Features
Real-time notifications for important changes
Clear visual displays for easy comprehension
Customizable settings to fit your specific needs
Integration with existing tools for a seamless experience
User-friendly interface for quick access
Potential Use Cases and Benefits
Monitor project updates to stay on track
Receive alerts for budget changes to manage finances efficiently
Track performance metrics to enhance team productivity
Stay informed about customer feedback to improve service
Evaluate risks to make proactive decisions
By using the Complete Equation Notice feature, you will solve common problems such as information overload and delayed responses. This feature ensures you stay updated without drowning in data. It helps you manage priorities, fosters timely responses, and enhances overall productivity.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do you find the complete ionic equation?
Start with a balanced molecular equation.
Break all soluble strong electrolytes (compounds with (a) beside them) into their ions. Indicate the correct formula and charge of each ion. Indicate the correct number of each ion. ...
Bring down all compounds with (s), (l), or (g) unchanged.
How do you find the ionic equation?
Start with a balanced molecular equation.
Break all soluble strong electrolytes (compounds with (a) beside them) into their ions. Indicate the correct formula and charge of each ion. Indicate the correct number of each ion. ...
Bring down all compounds with (s), (l), or (g) unchanged.
How do you find ionic equations?
Start with a balanced molecular equation.
Break all soluble strong electrolytes (compounds with (a) beside them) into their ions. Indicate the correct formula and charge of each ion. Indicate the correct number of each ion. ...
Bring down all compounds with (s), (l), or (g) unchanged.
What is an ionic equation?
The two most common forms of ionic equations are complete ionic equations and net ionic equations. The complete ionic equation indicates all the dissociated ions in a chemical reaction.
What is the net ionic equation of the reaction of?
The net ionic equation is a chemical equation for a reaction which lists only those species participating in the reaction. The net ionic equation is commonly used in acid-base neutralization reactions, double displacement reactions, and redox reactions.
What is the difference between an ionic equation and a half equation?
These are known as half-equations. The two half-equations combined give the overall equation. Ionic half-equation simply refers to the fact that we simplify the half-equation by only showing the ions that undergo change.
What is a total ionic equation?
Summary. The net ionic equation shows only the chemical species that are involved in a reaction, while the complete ionic equation also includes spectator ions. We can find the net ionic equation using the following steps: Write the balanced molecular equation, including the state of each substance.
What is the difference between a net ionic equation and a complete ionic equation?
Your complete ionic equation includes all ions in solution, including spectator ions. Your net ionic equation leaves out spectator ions and focuses on what changes in the reaction. I leave out sodium and chloride ions because they are irrelevant in the reaction and are called spectator ions.
How do you balance ionic equations?
Write the net ionic equation for the unbalanced reaction. ...
Separate the net ionic equation into the two half-reactions. ...
For one of the half-reactions, balance the atoms except for O and H. ...
Repeat this with the other half-reaction.
Add H2O to balance the O atoms. ...
Balance charge.
What is the net ionic equation for water?
H+ and OH. KHSO4 is water-soluble, so it will not form. However, H+ will bond to OH whenever the two are put together, so our product is H2O. The net ionic equation is: H+(a) + OH(a) H2O(l) Note that when water is involved in an aqueous reaction, it is always written H2O(l), not H2O(a).
#1 usability according to G2
Try the PDF solution that respects your time.






