Complete Equation Title For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
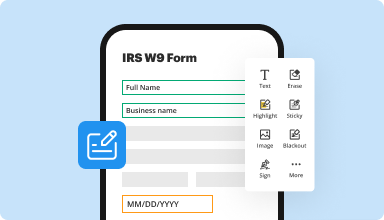
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
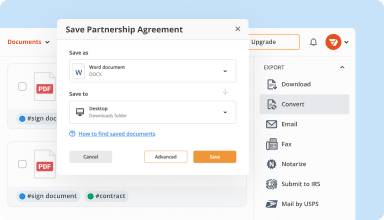
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
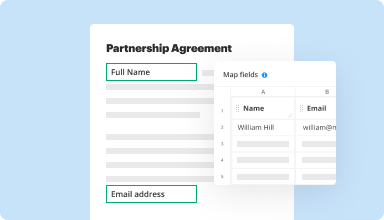
Collect data and approvals
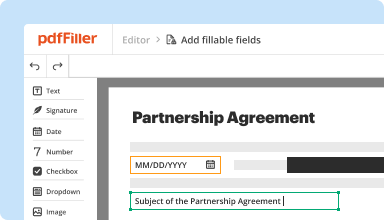
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
I completed the first PDF form and it was a little tough maneuvering through the documents, savings. It was a little disingenuous to wait until I was finished to document to find out that I would be billed annually as apposed to monthly, when the advertising gave the amount payable monthly.
2015-11-03
Been a life saver for my business brokerage and real estate businesses. It allows me to make simple corrections to send back out to buyers and sellers.
2017-07-02
Still on free trial period. Have used it twice and very easy to understand.
Filled in a large medical form of 6 pages and had no hassles
As I loaded it for my home use and probably will only use it occasionally I think the cost may be unjustifiable for me to continue with it which is a pity. But it is a good product
2020-03-28
Awesome and practical!
Awesome and practical !An easy process to fill your PDF forms, it saves your signature for your futur use.You won’t regret buying this program. You can always try it for free as I did myself. Customer service agents are very helpful and consistent to check that everything is well and mostly the customer is satisfied.Thank you Khadija B.
2019-04-16
Time saver!
I have used PDFfiller for several months now and love it. It is a great tool which makes it extremely easy for me to quickly and efficiently fill out PDFs and return them to the sender. Wether it be filling out forms or signing a contract, it has saved me many many hours in the long run - and is a pleasure to use!
There aren't really any cons to this plugin - it does exactly what it says it will do. However the user interface could be improved
2018-11-06
Well I am very computer savvy but there have been some challenges to learning pdffiller
I was creating fillable forms and then opening them on my Mac using Preview and the fillable fields wouldn't show. Then I opened with Adobe reader and everything was fine.
I would like to know if it is possible for me to create a pdf that my students could use their stylus to write and draw on?
2021-09-22
I signed up for a trial and forgot that…
I signed up for a trial and forgot that the annual subscription kicked in a month later. Subsequently, I realised that I have no use for the service/product. PDFfiller was kind and understanding. They responded almost immediately and refunded me without hesitation.
2020-08-30
Great service. I thought my subscription had expired but the tech support hooked me back up in a jiffy! I prefer to use PDFfiller program rather than the Acrobat Reader DC program. I will always renew my subscription in the future.
2020-05-19
I really appreciate being able to transform a regular, annoying PDF into an easily accessible document my clients can sign from any device and be sent by almost any app or service. This will definitely make going paperless a reality for our business
2020-04-30
Complete Equation Title Feature
Introducing the Complete Equation Title feature, designed to enhance your workflow and simplify complex tasks. This powerful tool helps you organize and present your equations clearly and concisely.
Key Features
Intuitive interface for easy navigation
Customizable templates for equations
Real-time collaboration options
Seamless integration with popular software
Support for multiple formats and outputs
Potential Use Cases and Benefits
Perfect for students needing to present their math homework
Ideal for professionals preparing reports or presentations
Useful for educators designing curriculum materials
Great for researchers drafting academic papers
Aids anyone looking to streamline their equation management
This feature effectively addresses challenges such as unclear presentations or cumbersome workflows. By using the Complete Equation Title, you can present your work confidently, reducing errors and saving you time. Whether you are a student, a professional, or an educator, this tool allows you to focus on what matters most—your content.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do you find the complete ionic equation?
Start with a balanced molecular equation.
Break all soluble strong electrolytes (compounds with (a) beside them) into their ions. Indicate the correct formula and charge of each ion. Indicate the correct number of each ion. ...
Bring down all compounds with (s), (l), or (g) unchanged.
How do you find the ionic equation?
Start with a balanced molecular equation.
Break all soluble strong electrolytes (compounds with (a) beside them) into their ions. Indicate the correct formula and charge of each ion. Indicate the correct number of each ion. ...
Bring down all compounds with (s), (l), or (g) unchanged.
How do you find ionic equations?
Start with a balanced molecular equation.
Break all soluble strong electrolytes (compounds with (a) beside them) into their ions. Indicate the correct formula and charge of each ion. Indicate the correct number of each ion. ...
Bring down all compounds with (s), (l), or (g) unchanged.
What is an ionic equation?
The two most common forms of ionic equations are complete ionic equations and net ionic equations. The complete ionic equation indicates all the dissociated ions in a chemical reaction.
What is the net ionic equation of the reaction of?
The net ionic equation is a chemical equation for a reaction which lists only those species participating in the reaction. The net ionic equation is commonly used in acid-base neutralization reactions, double displacement reactions, and redox reactions.
What is the difference between an ionic equation and a half equation?
These are known as half-equations. The two half-equations combined give the overall equation. Ionic half-equation simply refers to the fact that we simplify the half-equation by only showing the ions that undergo change.
What is a total ionic equation?
Summary. The net ionic equation shows only the chemical species that are involved in a reaction, while the complete ionic equation also includes spectator ions. We can find the net ionic equation using the following steps: Write the balanced molecular equation, including the state of each substance.
What is the difference between a net ionic equation and a complete ionic equation?
Your complete ionic equation includes all ions in solution, including spectator ions. Your net ionic equation leaves out spectator ions and focuses on what changes in the reaction. I leave out sodium and chloride ions because they are irrelevant in the reaction and are called spectator ions.
How do you balance ionic equations?
Write the net ionic equation for the unbalanced reaction. ...
Separate the net ionic equation into the two half-reactions. ...
For one of the half-reactions, balance the atoms except for O and H. ...
Repeat this with the other half-reaction.
Add H2O to balance the O atoms. ...
Balance charge.
What is the net ionic equation for water?
H+ and OH. KHSO4 is water-soluble, so it will not form. However, H+ will bond to OH whenever the two are put together, so our product is H2O. The net ionic equation is: H+(a) + OH(a) H2O(l) Note that when water is involved in an aqueous reaction, it is always written H2O(l), not H2O(a).
#1 usability according to G2
Try the PDF solution that respects your time.






