Consent Signature For Free




Join the world’s largest companies
How to Send a PDF for eSignature









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Consent Signature Feature
The Consent Signature feature streamlines the process of obtaining and managing signatures for various agreements. It enhances your workflow, ensuring compliance and accountability while providing a clear record of consent.
Key Features
Potential Use Cases and Benefits
With the Consent Signature feature, you solve the common problems of delayed approvals and lost documents. It simplifies the process of gaining consent, making it faster and more reliable. By using this feature, you can ensure that all parties are on the same page, thus improving trust and satisfaction in your transactions.
Consent Signature in minutes
pdfFiller enables you to Consent Signature quickly. The editor's convenient drag and drop interface ensures fast and user-friendly signing on any device.
Ceritfying PDFs electronically is a fast and secure way to validate paperwork anytime and anywhere, even while on the fly.
See the step-by-step guide on how to Consent Signature electronically with pdfFiller:
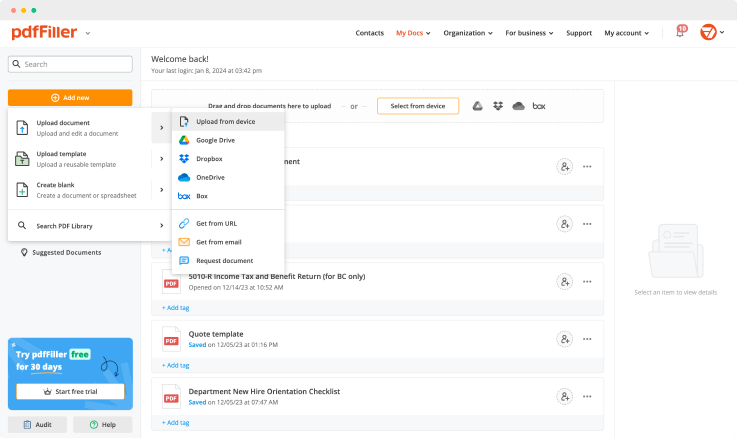
Upload the document you need to sign to pdfFiller from your device or cloud storage.
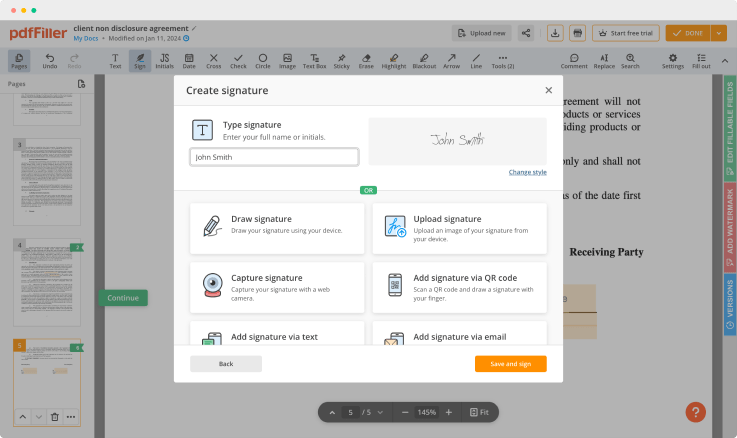
As soon as the document opens in the editor, click Sign in the top toolbar.
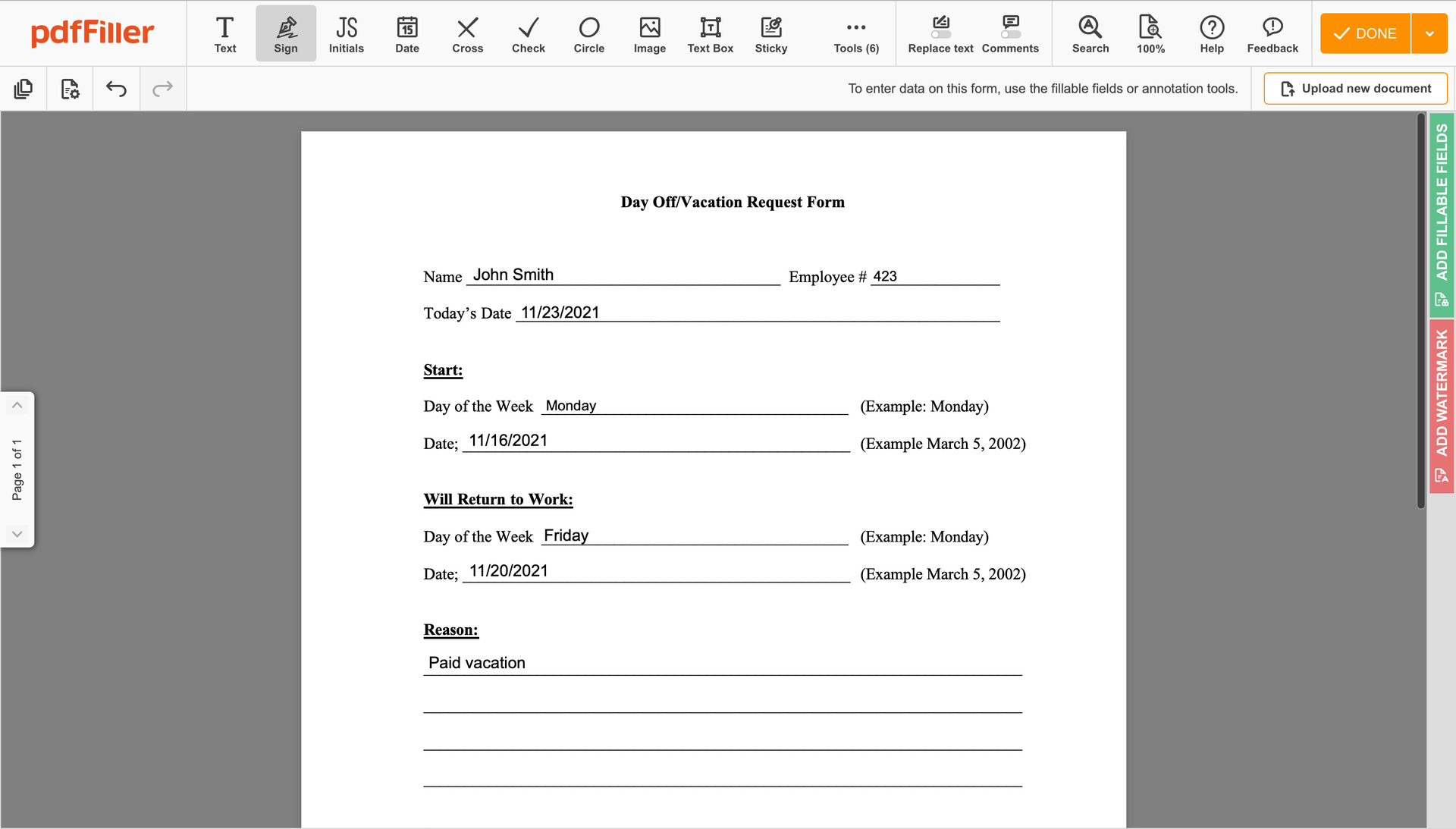
Create your electronic signature by typing, drawing, or uploading your handwritten signature's photo from your device. Then, click Save and sign.
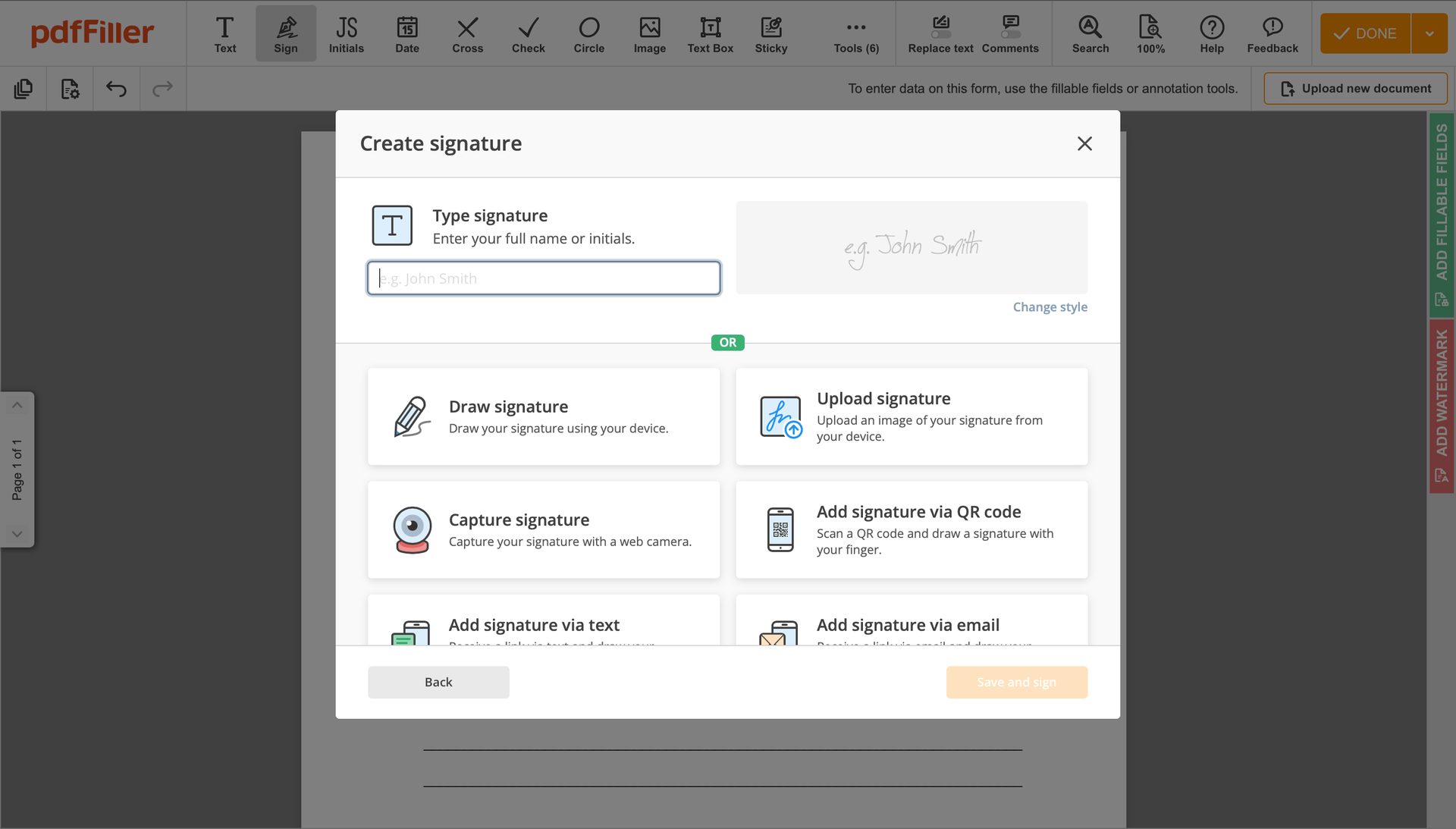
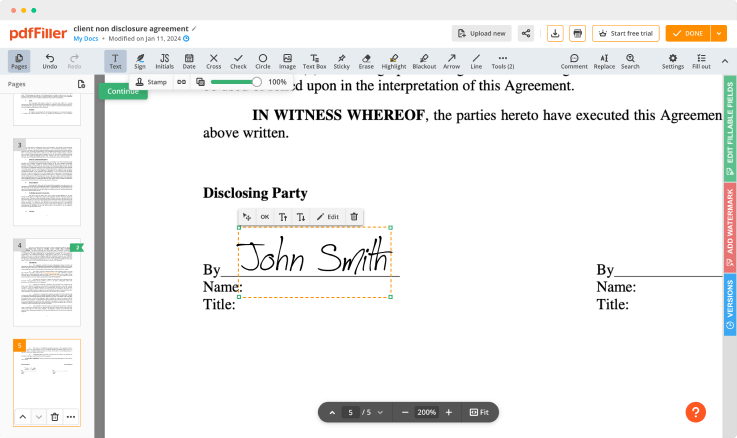
Click anywhere on a document to Consent Signature. You can move it around or resize it utilizing the controls in the hovering panel. To apply your signature, click OK.
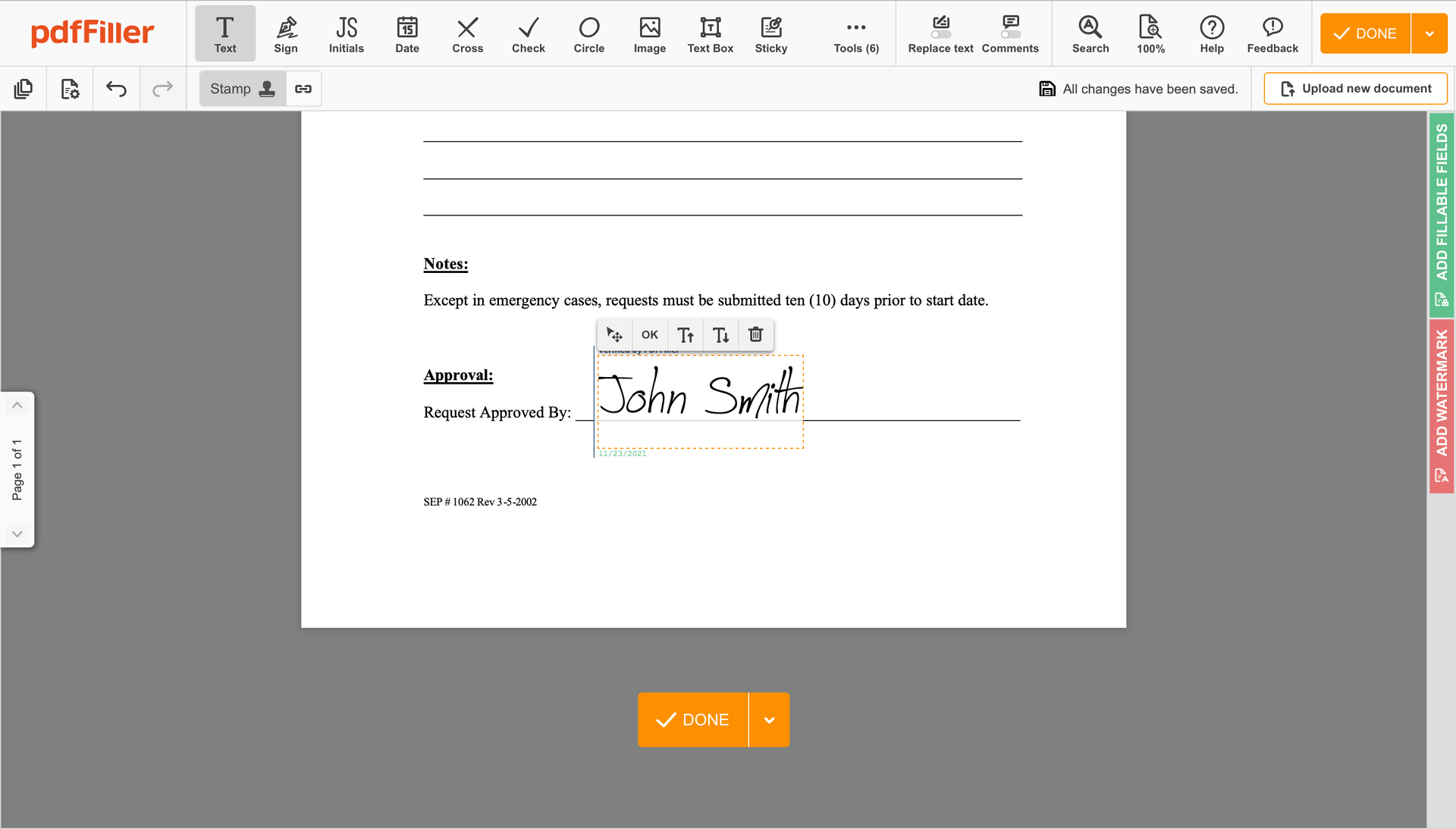
Finish up the signing process by clicking DONE below your form or in the top right corner.
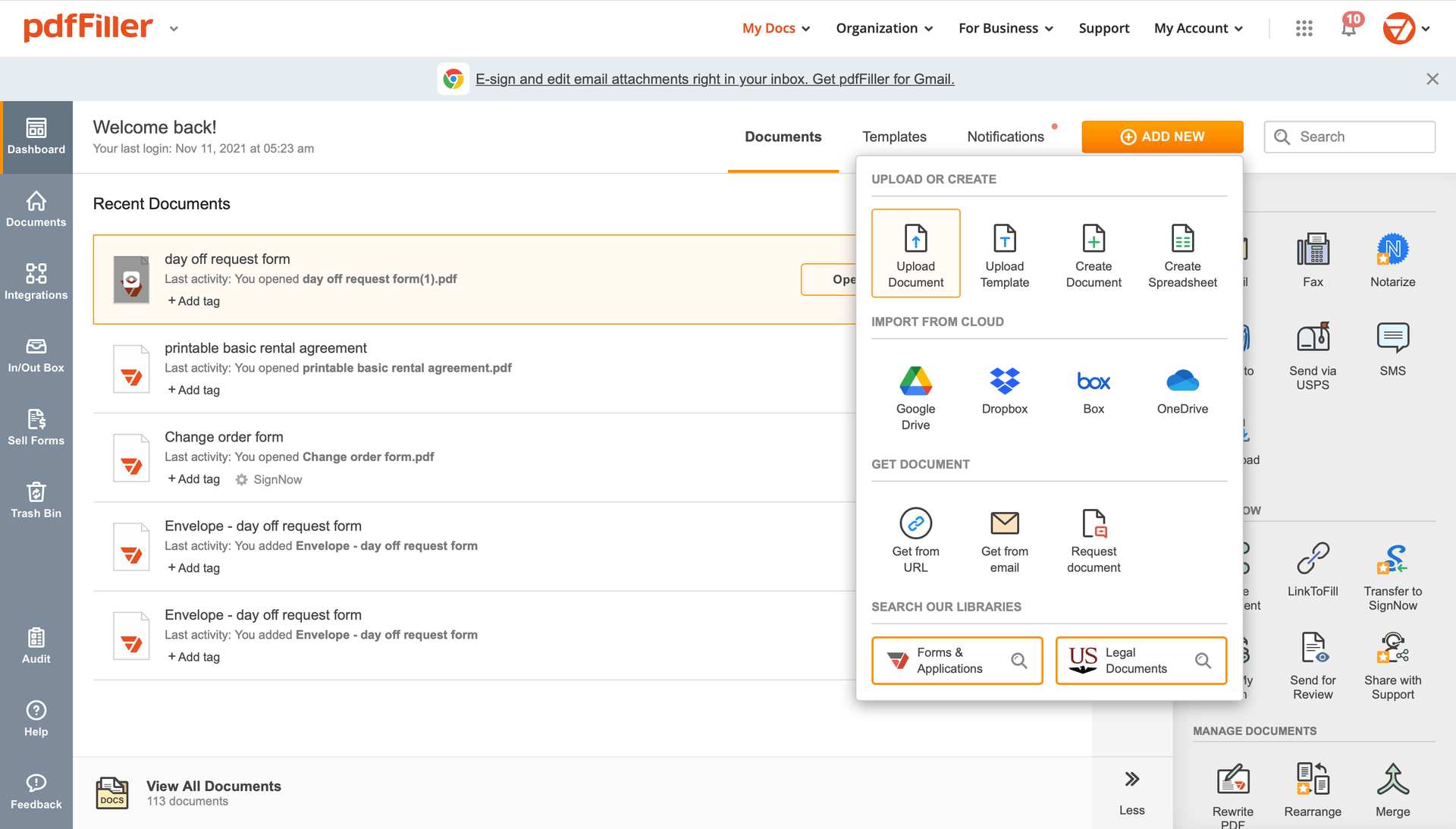
After that, you'll go back to the pdfFiller dashboard. From there, you can get a completed copy, print the form, or send it to other people for review or approval.
Still using different applications to manage your documents? Try our solution instead. Use our editor to make the process simple. Create document templates on your own, edit existing forms, integrate cloud services and other features without leaving your account. You can Consent Signature directly, all features are available instantly. Pay as for a lightweight basic app, get the features as of a pro document management tools.
How to edit a PDF document using the pdfFiller editor:
For pdfFiller’s FAQs
Ready to try pdfFiller's? Consent Signature