Control Bookmark Accreditation For Free
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

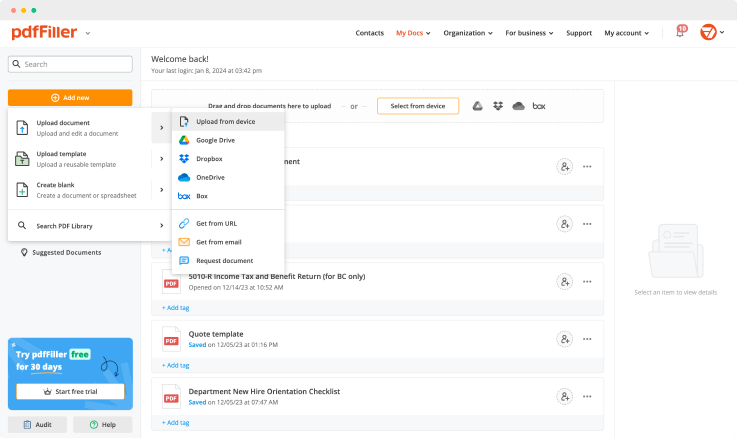
Upload a document

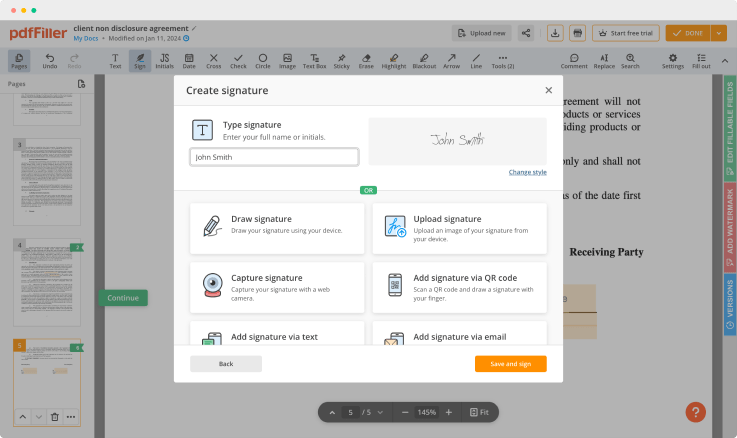
Generate your customized signature

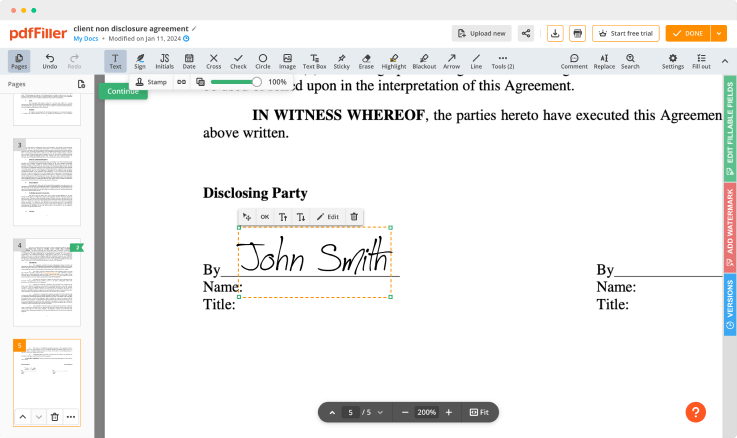
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Control Bookmark Accreditation Feature
The Control Bookmark Accreditation feature helps you manage and verify your bookmarks with ease. This tool ensures that your saved links meet quality standards, providing you with a reliable way to access important resources.
Key Features
User-friendly interface for easy navigation
Real-time verification of bookmark credibility
Customizable accreditation criteria to fit your needs
Analytics dashboard to track bookmark performance
Seamless integration with existing tools and platforms
Potential Use Cases and Benefits
Individuals streamlining their personal research efforts
Teams ensuring consistent quality in shared resources
Educational institutions verifying student reference materials
Businesses maintaining a reliable content repository
Content creators managing affiliate and reference links
With the Control Bookmark Accreditation feature, you can confidently navigate the web. It solves the problem of unreliable bookmarks by offering a way to ensure that each one is credible and useful. By using this feature, you save time and enhance your productivity, allowing you to focus on what truly matters.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What is IPA accreditation?
The Medicare Improvements for Patients and Providers Act (MI PPA) calls for all providers of CT, MRI, breast MRI, nuclear medicine, and PET exams that bill under Part B of the Medicare Physician Fee Schedule to be accredited by Jan. 1, 2012, in order to receive payment for the technical component of these services.
What is a CLI AID number?
Congress passed the Clinical Laboratory Improvement Amendments, or CIA, in 1988 in an attempt to standardize testing and provide oversight for all laboratory testing done in the United States. ... The certificate will include a 10-digit number, which is your CIA number.
How do I get a CIA number?
How do I apply for a CIA certificate? The CIA application (Form CMS-116) is available online. Send your completed application to the address of the local State Agency for the State in which your laboratory is located. Additionally, check with your State Agency for any other state- specific requirements.
How do I get my CIA card?
Must have completed CIA's State of the Industry Course located on the CIA site under the Training tab and click on Online Learning Courses
Must have completed KHM Travel Group's Getting Started Agent Basics Education.
What is a CIA number?
Congress passed the Clinical Laboratory Improvement Amendments, or CIA, in 1988 in an attempt to standardize testing and provide oversight for all laboratory testing done in the United States. ... The certificate will include a 10-digit number, which is your CIA number.
How do I get an IATA number?
Ensure you meet the local criteria as specified in the 'Travel Agent Handbook'
Select your country of application below and read carefully the Application Guide.
Submit your Application as specified in the Application Guide for your country
How much does a CIA card cost?
Dues for individual CIA memberships will drop from $119 a year to either $99 or $49. Fees at the agency level will remain unchanged at $339 annually for companies that employ less than 25 agents.
What is CIA and what is its purpose?
In general terms, the CIA regulations establish quality standards for laboratory testing performed on specimens from humans, such as blood, body fluid and tissue, for the purpose of diagnosis, prevention, or treatment of disease, or assessment of health.
What is the definition of CIA?
The Clinical Laboratory Improvement Amendments (CIA) of 1988 are United States federal regulatory standards that apply to all clinical laboratory testing performed on humans in the United States, except clinical trials and basic research.
Who needs a CIA certificate?
CIA requires that any facility examining human specimens for diagnosis, prevention, treatment of a disease or for assessment of health must register with the federal Centers for Medicare & Medicaid Services (CMS) and obtain CIA certification.
Ready to try pdfFiller's? Control Bookmark Accreditation
Upload a document and create your digital autograph now.