Draft Signature Record For Free




Join the world’s largest companies









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Draft Signature Record Feature
The Draft Signature Record feature allows users to easily manage and streamline their document-signing processes. This tool helps you maintain clear records while ensuring that every signature is captured precisely.
Key Features
Potential Use Cases and Benefits
By using the Draft Signature Record feature, you eliminate the hassle of manual signature tracking. The system answers your need for organization and efficiency, ensuring all signatures are documented and verified without delay. Say goodbye to lost documents and hello to a smoother workflow.
Instructions and Help about Draft Signature Record For Free
Draft Signature Record: easy document editing
Document editing has become a routine task for all those familiar to business paperwork. You're able to edit a Word or PDF file, thanks to different tools that allow applying changes to documents. At the same time, such apps take up space while reducing its performance. There are also lots of online document editing tools which work better on older devices and faster to use.
The good news is, now you can get just one tool to cover all the PDF needs to start working on documents online.
Using pdfFiller, you can store, change, produce, sign and send PDF documents online, without leaving a single browser tab. The platform supports PDF documents and other common file formats, such as Word, images, PowerPoint and much more. Upload documents from your device and start editing in just one click, or create new form yourself. pdfFiller works across all devices with active internet connection.
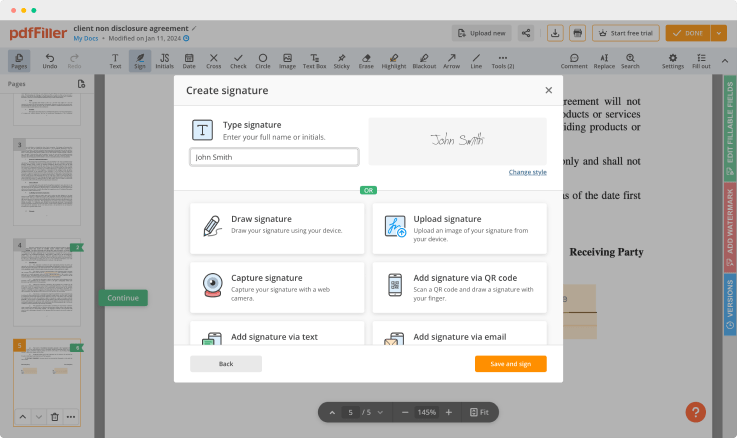
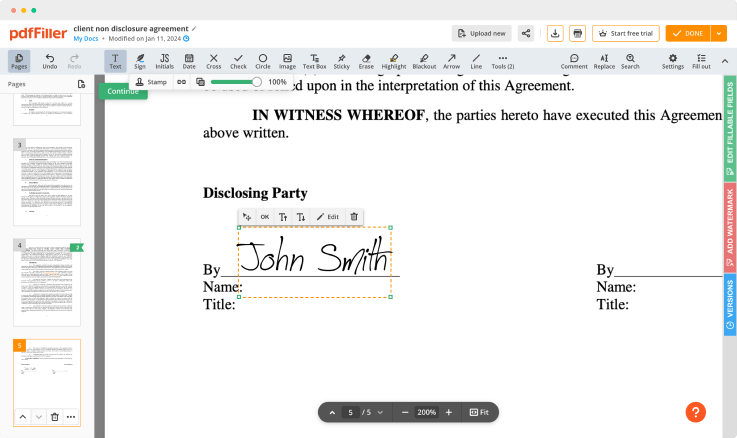
pdfFiller comes with a multi-purpose text editor to rewrite the content of your document. There is a great selection of tools for you to modify not only the document's content but its layout, to make it appear more professional. Edit pages, add fillable fields anywhere on the document, add images and spreadsheets, customize the text formatting and put digital signature — all in one place.
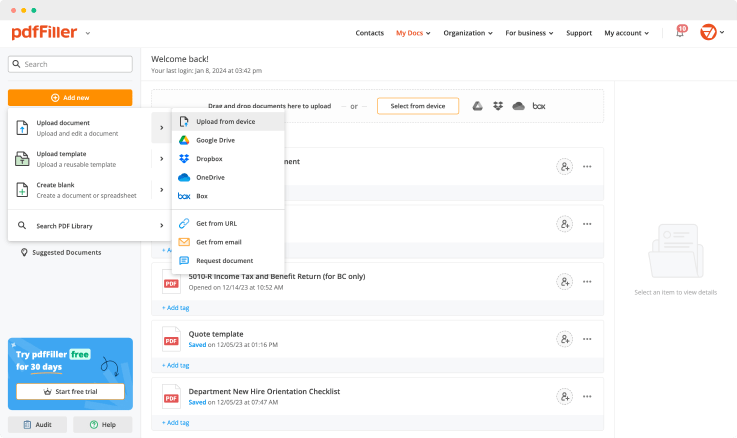
Use one of the methods below to upload your document and start editing:
As soon as your document is uploaded, it's saved to the Docs folder instantly. pdfFiller stores your data encrypted and on remote server, to provide you with extra level of security. This means that they cannot be lost or accessed by anyone but yourself and users you share your document with. Move all the paperwork online and save time.
For pdfFiller’s FAQs
Ready to try pdfFiller's? Draft Signature Record