Fillable Signature Notification For Free




Join the world’s largest companies
How to Fillable Signature Notification - video instructions
Watch the video guide to learn more about pdfFiller's online Signature feature









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Fillable Signature Notification Feature
The Fillable Signature Notification feature enhances your document workflow by allowing you to collect signatures efficiently. Simplify your process while ensuring security and compliance.
Key Features:
Potential Use Cases and Benefits:
This feature addresses common challenges like delays in obtaining signatures and managing document versions. With the Fillable Signature Notification feature, you can increase efficiency, reduce errors, and improve satisfaction for both your team and clients.
Instructions and Help about Fillable Signature Notification For Free
Fillable Signature Notification: make editing documents online a breeze
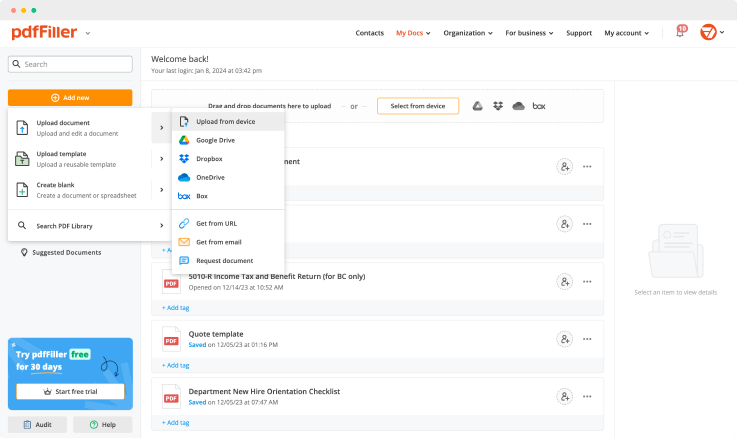
If you've ever had to fill out an affidavit or application form in really short terms, you are aware that doing it online is the most convenient way. Filling out is a breeze, and you are able to forward it to another person right away. Having access to a PDF editor gives you the opportunity to edit text, add images, complete forms and convert PDF to other formats.
With pdfFiller, add text, sheets, images, checkmarks, edit existing content or create entirely new documents. Once finished, save it as a PDF file, or export to the program you're using with built-in integration's features. With pdfFiller, any PDF document can be converted into Word, PowerPoint, image or spreadsheet.
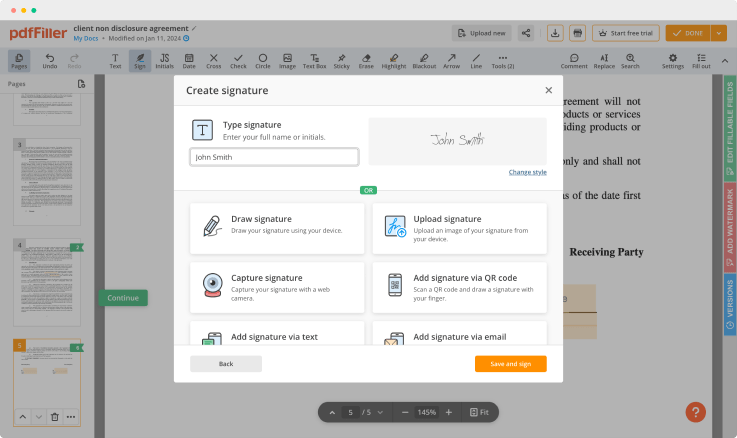
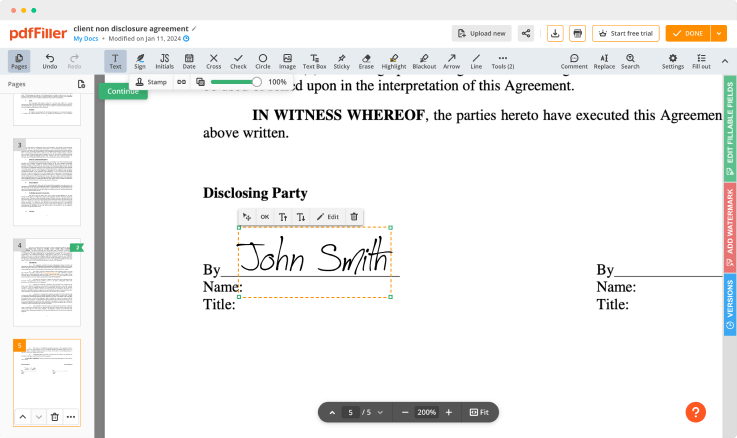
Create legally binding signatures from a photograph, with e-signing feature. Get access to this from all your devices and your signature will be verified all across the United States, under the DESIGN Act of 2000. Upload an existing digital signature from your computer, or use QR codes for verifying documents.
Discover the numerous features for editing and annotating PDF files on the go. Store your information securely and access across all your devices using cloud storage.
Edit. Make changes to your documents with a user-friendly interface. Add images, watermarks and checkmarks. Highlight or blackout the particular text
Create documents from scratch. Add fillable fields. Add and erase text.
Fill out forms. Select from the range of forms and select the one you are looking for
Protect with password. Prevent third parties from unauthorized access to your data
Change the format. Convert PDF files to any document format including Word, Excel, Google Docs, Pages and more
For pdfFiller’s FAQs
Ready to try pdfFiller's? Fillable Signature Notification