Fillable Signature Record For Free




Join the world’s largest companies
How to Fillable Signature Record - video instructions
Watch the video guide to learn more about pdfFiller's online Signature feature









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Fillable Signature Record Feature
Introducing the Fillable Signature Record feature, designed to streamline your documentation process. This tool simplifies the way you handle signatures, making it easier for you and your clients to manage important agreements and records.
Key Features
Potential Use Cases and Benefits
The Fillable Signature Record feature solves your documentation challenges by providing a clear, efficient solution that eliminates the need for printing and scanning. This tool enhances your workflow by allowing you and your clients to sign documents from anywhere, reducing delays and boosting productivity. Embrace the future of paperwork and make every signature count.
Instructions and Help about Fillable Signature Record For Free
Fillable Signature Record: full-featured PDF editor
Since PDF is the most popular document format in business, the right PDF editor is vital.
Even if you hadn't used PDF for your business documents before, you can switch anytime — it is easy to convert any other file format into PDF. Several file formats containing various types of content can also be combined within one glorious PDF. Using PDF, you can create presentations and reports that are both comprehensive and easy-to-read.
Though numerous online solutions provide PDF editing features, only a few of them allow adding signatures, collaborating with other users etc.
pdfFiller’s powerful editing solution has features for editing, annotating, converting PDFs into other formats, adding electronic signatures, and filling out PDF forms. pdfFiller is an online PDF editor you can use in your browser. You don’t have to install any applications. It’s a complete solution you can use from any device with an internet connection.
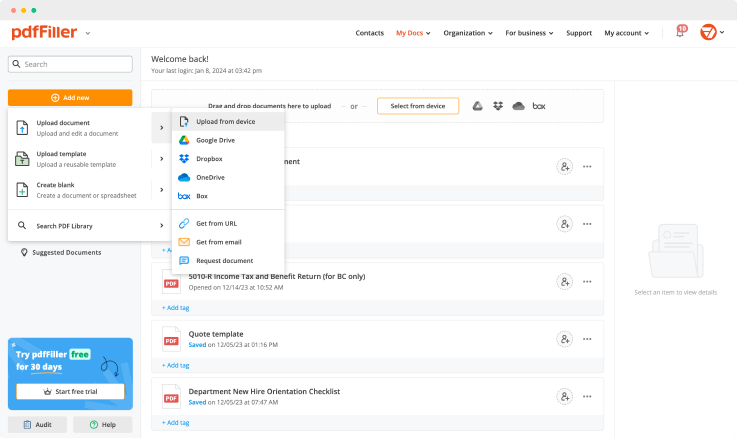
Use one of the methods below to upload your document and start editing:
Once you uploaded the document, it’s saved in the cloud and can be found in the “My Documents” folder.
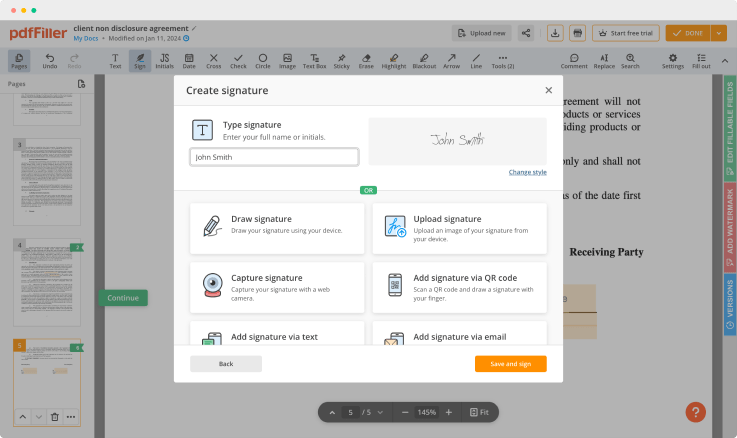
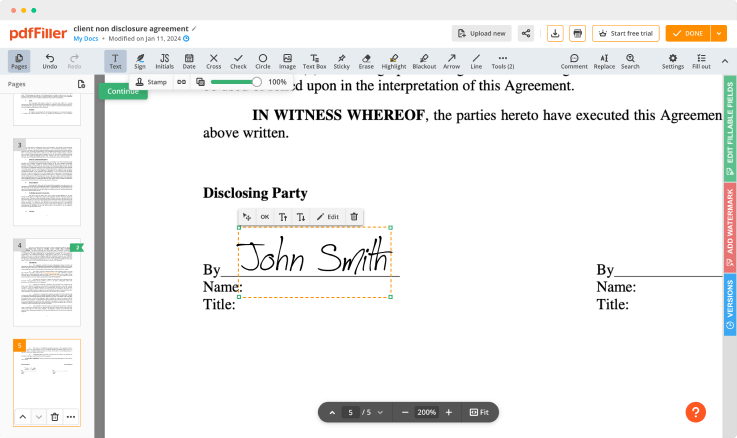
Use editing tools to type in text, annotate and highlight. Add fillable fields and send documents for signing. Change a document’s page order. Add images into your PDF and edit its appearance. Collaborate with other people to complete the fields and request an attachment if needed. Once a document is completed, download it to your device or save it to the third-party integration cloud.
For pdfFiller’s FAQs
Ready to try pdfFiller's? Fillable Signature Record