Fit Signature Record For Free




Join the world’s largest companies









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Fit Signature Record Feature
The Fit Signature Record feature streamlines your fitness journey. It provides an effective way to track personal achievements and monitor progress. This tool makes it easy for you to maintain motivation and set new goals.
Key Features
Use Cases and Benefits
In summary, the Fit Signature Record feature effectively addresses the challenge of monitoring fitness progress. By providing clear data and insightful reports, it helps you stay on track and motivated. This way, you can focus on your fitness journey with confidence.
Instructions and Help about Fit Signature Record For Free
Fit Signature Record: easy document editing
Using the best PDF editing tool is important to enhance your document management.
All the most widely used document formats can be easily converted into PDF. This makes creating and sharing most of them effortless. Several file formats containing different types of data can be combined within just one PDF. Using PDF, you can create presentations and reports that are both comprehensive and easy-to-read.
Though there are many solutions offering PDF editing features, it’s hard to find one that covers the range of the features available on the market at a reasonable cost.
Use pdfFiller to annotate documents, edit and convert into many other formats; fill them out and add an e-signature, or send out to others. All you need is in the same browser window. You don’t need to install any programs.
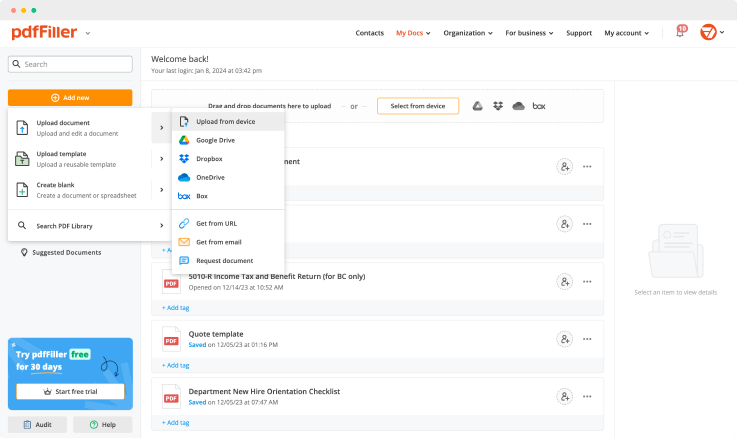
Create a document yourself or upload an existing one using the next methods:
Once you uploaded the document, it’s saved and can be found in the “My Documents” folder.
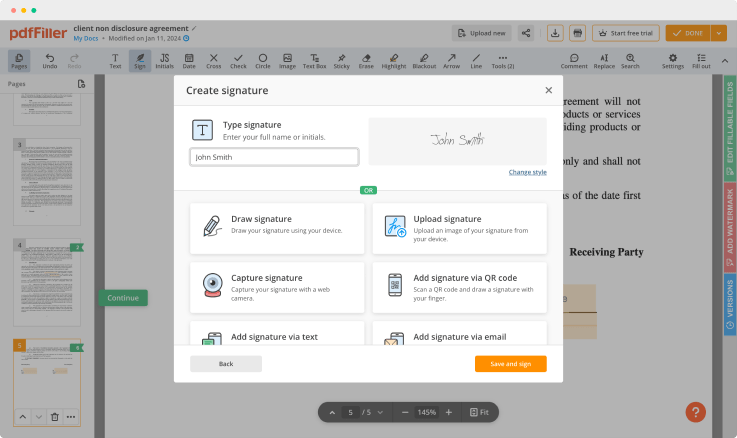
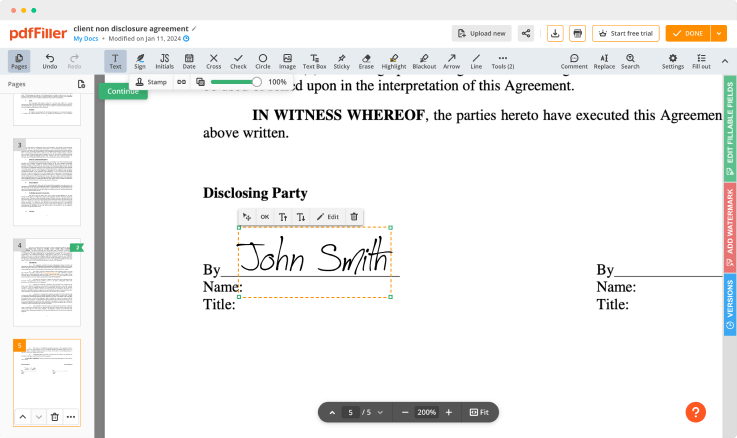
Use editing features to type in text, annotate and highlight. Add and edit visual content. Change a document’s page order. Add fillable fields and send to sign. Ask your recipient to fill out the document and request an attachment. Once a document is completed, download it to your device or save it to the third-party integration cloud.
For pdfFiller’s FAQs
Ready to try pdfFiller's? Fit Signature Record