Group Period Form For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
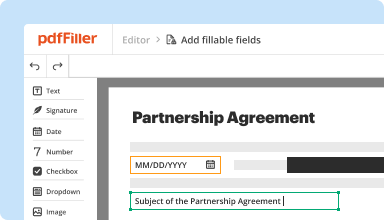
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
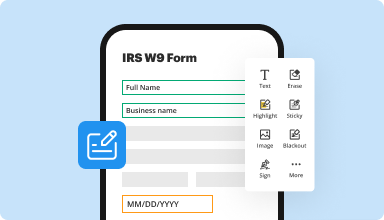
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
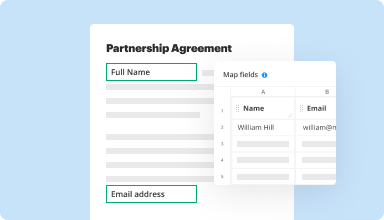
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
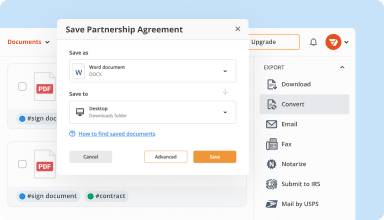
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
It is a wonderful program to use now that I have figured out what today. It is very easy to move around in the program too. Thank you for the intro videos too!
2015-06-22
So easy to use.....I was in need of a Quitclaim Deed for mineral rights, and PDFfiller was the only site that offered the form that I needed. What a find!
2015-08-19
Easy to use but should advise up front that paid membership is required as once document is completed a person is pretty much obligated if they want to send or print...
2016-03-31
Quick and easy editing features. Not sure how to utilized the signing of documents feature, but will figure it out. I also would like to delete old docs no longer needed. An online class for the software use would be appreciated.
2018-04-30
Very useful service. Trying to create a fillable pdf is made simple. Although when it's downloaded, one or two areas are not fillable anymore so have to do it again.
2019-02-11
What do you like best?
This is available everywhere where you go. You do not have to take your computer or have a software installed on your laptop. You can access it from anywhere else. In addition, it is very user friendly to navigate through the platform to accomplish what you would like to do.
What do you dislike?
I just don't have any dislike. So there is not much to say for this area.
Recommendations to others considering the product:
It is easy and available for access anywhere you access a computer. You do not have to have one.
What problems are you solving with the product? What benefits have you realized?
All my PDF applications are done through the platform. Fast, available always, and you can do it on any computer or mobile device.
This is available everywhere where you go. You do not have to take your computer or have a software installed on your laptop. You can access it from anywhere else. In addition, it is very user friendly to navigate through the platform to accomplish what you would like to do.
What do you dislike?
I just don't have any dislike. So there is not much to say for this area.
Recommendations to others considering the product:
It is easy and available for access anywhere you access a computer. You do not have to have one.
What problems are you solving with the product? What benefits have you realized?
All my PDF applications are done through the platform. Fast, available always, and you can do it on any computer or mobile device.
2019-01-02
Intuitive & straightforward
This online software seems pretty intuitive to use and I've had a good experience of collecting signatures online, so far.
2022-11-23
Fast! We love the way it transfers previous, redundant info...
Very Accurate...woo hoo! Thank you, worth every bit the price, especially if everything submits electronically.
2021-01-29
I found operator Dee skilled and…
I found operator Dee skilled and totally switched On to my problem. No beating around the bush - my problem was resolved in under 5m of the chat.It is refreshing and lucky to meet such Customer Service professionals these.Well done Dee - Thanks much for your assist.CheersYaz
2020-12-21
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do you find the group and period?
The period of an element corresponds to the principal quantum number of the Valence shell. The block of an element corresponds to the type of orbital which receive the last electron. For s-block elements, group number is equal to the number of valence electrons.
How do you find the group and period of an element?
The period of an element corresponds to the principal quantum number of the Valence shell. The block of an element corresponds to the type of orbital which receive the last electron. For s-block elements, group number is equal to the number of valence electrons.
How do you find the group and period of an element in class 10?
Valence Electron — Group 1. Valence Electrons — Group 2. To 8 valence electrons — Add 10 to the valence electrons to get the group number of the element.
What is group and period in periodic table?
Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period.
How do you find the electron configuration of a group?
The group of an element is predicted from the number of electrons in the valence shell or/and penultimate shell as follows: a)For s block elements, group number is equal to the number of valence electrons. b) For p block elements, group number is equal to 10+number of electrons in the valence shell.
What do periods and groups tell you?
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods.
How do you find the group number of an element?
For s-block elements, group number is equal to the number of valence electrons. For p-block elements, group number is equal to 10+number of electrons in the Valence shell. For d-block elements group number is equal to the number of electrons in a (n-1) d subshell + the number of electrons in Valence shell.
How do you determine the period and group of an electron configuration?
The number of valence electrons in the atom determines the group number of that atom i.e. if an element has 4 valence electrons then it must belong to group IV A. Since sodium has 3 shells, it belongs to period 3. Also, we notice that this element has 1 electron in its outermost orbit therefore it belongs to group I Am.
#1 usability according to G2
Try the PDF solution that respects your time.






