Insert Table in the Clinical Trial Agreement Template with ease For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
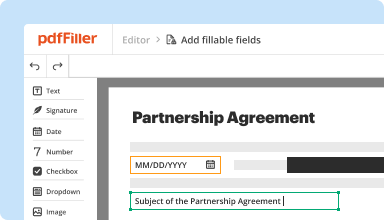
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
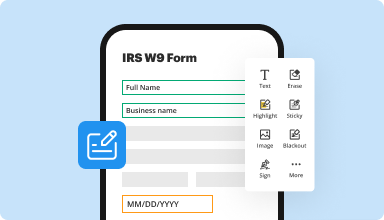
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
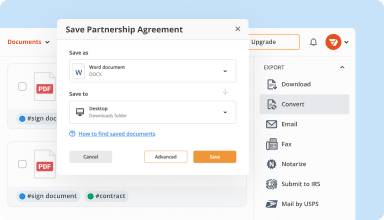
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
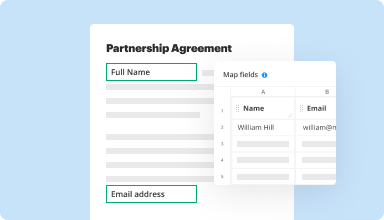
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
Was looking for a fast PDF fillable form. I found it here. Even has e-sign which really makes me look professional. Always wanted to try it and now I'm glad I did.
2016-05-27
It was the worst because I had no idea how to work the program. It took well over 5hrs for something that should have taken less than 5 mins had a I known what to do
2018-06-06
PDFfiller Fills Needs
My overall experience has been positive and if the cons are minimal.
It's easy to use and allows me to make necessary additions or changes to documents with a minimum of fuss.
Sometimes getting the font to match is a little challenging, but it's easier than trying to "fix," documents with white out and hand print information.
2019-05-24
PSFfiller is a very accommodating…
PSFfiller is a very accommodating company. Their customer service is really fast and helpful. All questions and concerns are dealt with immediately. They go the extra mile for their customers.
2024-04-15
used a handful of other pdf programs, and while there have been a decent one here or there, most have been quite irresponsible, irresponsive, and irritable! pdfFiller is by far the most user-friendly, quick, and manageable one there is, notes this observer! am definitely glad i went for the platinum membership - has not only calmed my disdain towards pdfs, but i am actually having fun with them now! ^_^
salamat poh, y arrigato, mi llamato ~
2022-11-14
I really like your product. I am not very computer savvy so I would greatly appreciate a webinar to help with navigating your website. That being said I have used your chat feature several times and your agents have all been awesome...very helpful!!
2022-09-01
I love this app...exactly what I was…
I love this app...exactly what I was looking for and it offers more than what I need but will be able to use for my future Business Needs!
2021-06-17
Not very user friendly
Not very user friendly. The "sign-up" commitment is not good... Inevitably some people will forget to cancel and then will be hit with a high annual charge...
2021-02-04
Combining two files was relatively easy once I read...
Combining two files was relatively easy once I read the How to Guide three times to figure it out. But editing text in the file resulted in a change in the font that was not particularly satisfying and could not be corrected.
2020-06-10
Insert Table in the Clinical Trial Agreement Template Feature
The Insert Table feature in the Clinical Trial Agreement Template streamlines the process of adding structured data to your agreements. With this tool, you can enhance clarity and organization in your documents, making it easier for all parties to review critical information.
Key Features
Easily insert tables for study protocols
Customize table layouts to fit specific needs
Maintain data integrity with automatic formatting
Support for collaboration among team members
Potential Use Cases and Benefits
Include participant demographics in a clear format
Summarize trial timelines for better project management
Present budget breakdowns transparently
Facilitate quick reference for compliance and regulatory needs
By using the Insert Table feature, you can address the challenge of presenting complex data in a digestible format. This tool allows you to enhance communication and understanding among stakeholders, leading to quicker decisions and improved project outcomes.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
Who signs an agreement?
Signatories sign legal documents, international agreements, and contracts. These types of documents have multiple parties that need to sign the agreement. Signers are anyone who needs to provide a signature to legal documents.
Who signs the clinical protocol?
The final protocol should be signed off by the Chief Investigator as a minimum, but usually other signatures may be required such as those from the sponsor and trial statistician.
Who signs a clinical trial agreement?
Clinical Trial Agreements (CTAs) require signatures from both the trial sponsor and a University institutional official (IO) with signature authority delegated by the Board of Trustees of the Leland Stanford Junior University.
Who are the parties to a clinical trial agreement?
Parties to clinical trial or study agreements include the participating site, the study sponsor, and/or the relevant clinical research organization (CRO).
Who is responsible for registering a clinical trial?
The sponsor is responsible for registering the trial. In cases where there is no sponsor, investigators involved in the research must work with each other to identify a responsible party and ensure the trial is registered only once for the entire project.
What is a CTA in a clinical trial?
Definition. A clinical trial agreement (CTA) or clinical study agreement (CSA) is a legally binding agreement that governs the conduct of a particular study and sets forth the obligations of each party to the agreement.
Who are the parties to a clinical trial?
Sponsor: The person or group of people who supervise or fund the trial—such as a drug company. Participant: A person who volunteers to take part in the clinical trial—such as yourself! Investigator: A researcher who helps conduct the clinical trial—such as a doctor.
#1 usability according to G2
Try the PDF solution that respects your time.






