List Signature Record For Free




Join the world’s largest companies









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
List Signature Record Feature
The List Signature Record feature helps you track and manage signatures efficiently. It allows you to maintain a clear record of signed documents, making your workflow smooth and organized. You can rely on this feature to simplify document management and enhance accountability.
Key Features
Potential Use Cases and Benefits
This feature addresses common challenges like lost documents, disorganized records, and time-consuming signature retrieval. By offering a centralized solution, it ensures you always have access to needed signatures, supporting your operations and saving valuable time.
Instructions and Help about List Signature Record For Free
List Signature Record: make editing documents online simple
At some point in time, almost everyone has ever needed to file a PDF document. For example, an affidavit or application form that you need to fill out online. In case collaborate on PDFs with other people, and especially if you need to ensure the accuracy and precision of the information you are sharing, use PDF editing tools. You only need a PDF editing tool to make changes to your document: rewrite the text or add some more, attach media or fillable fields.
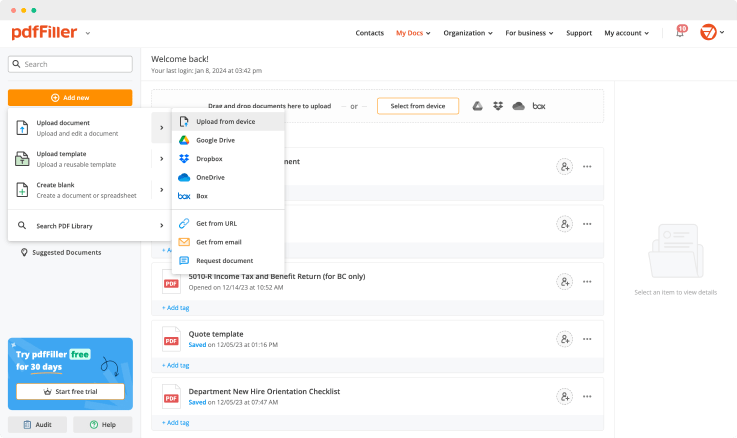
With pdfFiller, create new fillable template from scratch, or upload an existing one to adjust text, add sheets, pictures and checkboxes. When finished, save it as a PDF file, or export to the platform you're using with built-in integration's features. Convert PDFs to Excel spreadsheets, pictures, Word files and more.
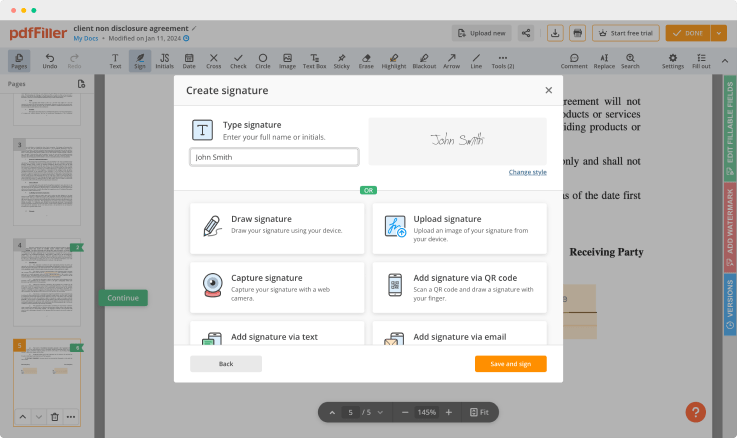
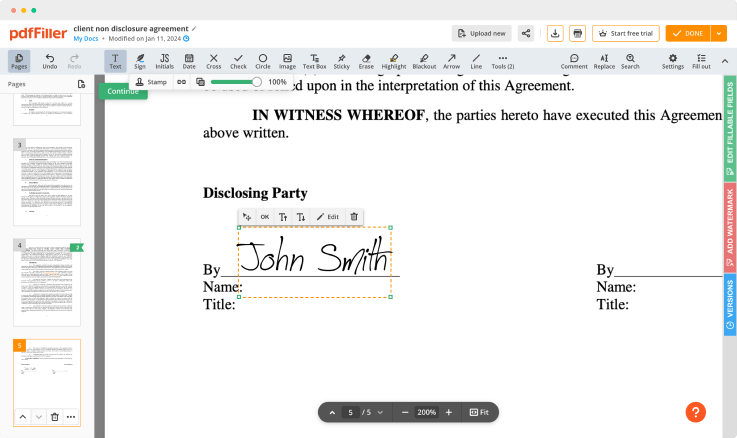
Create a unique signature using your mouse, touchpad, or upload it from a photo and attach it to documents. This functionality is available on both desktop and mobile devices, and is verified in all states (under the E-Sign Act of 2000).
Discover powerful editing features to make your documents look professional. Store your data securely and access across all your devices using cloud storage.
Edit. Change the content or mix it up with images, apply watermarks or add checkboxes
Create documents from scratch. Add and edit text, signature field, checkboxes and more
Fill out forms. Select from the range of ready-made forms and select the one you are looking for
Provide safety. Prevent others from unauthorized access to your data
Change the format. Convert PDF files to any document format including Word, Excel, Google Docs, Pages and more
For pdfFiller’s FAQs
Ready to try pdfFiller's? List Signature Record