Lock Up Signature Accreditation For Free
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

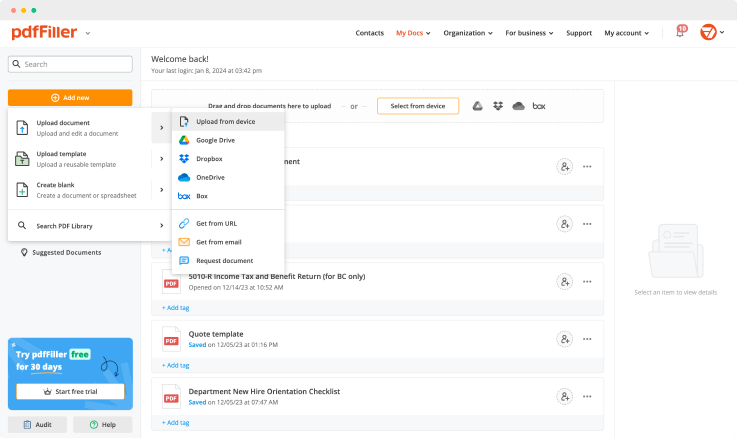
Upload a document

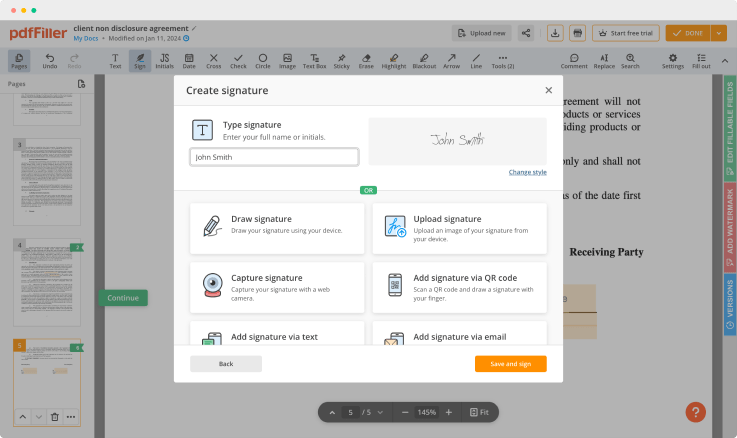
Generate your customized signature

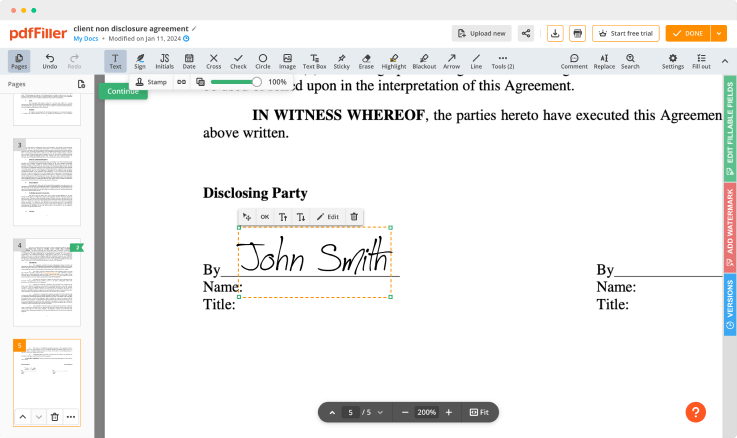
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Lock Up Signature Accreditation Feature
Lock Up Signature Accreditation is designed to provide your business with a secure, reliable, and efficient way to manage signatures. This feature enhances your document workflow, ensuring that every signature is authentic and verified.
Key Features of Lock Up Signature Accreditation
Real-time signature verification process
Secure cloud storage for documents
User-friendly interface for easy navigation
Comprehensive audit trails for accountability
Compatibility with various document formats
Potential Use Cases and Benefits
Streamlining contract signing for remote teams
Enhancing security for sensitive documents
Facilitating compliance in regulated industries
Reducing turnaround time for agreements
Improving client trust through secure transactions
By implementing Lock Up Signature Accreditation, you can solve common issues such as delayed document approvals and security concerns. This feature ensures that your signatures are captured accurately and securely, allowing you to focus on your business objectives with peace of mind.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do I lock my PDF signature?
Choose a Signature from the Sign As menu.
Enter the Password for the selected digital signature.
Select an Appearance, or choose To create New Appearance.
Enable Lock Document After Signing if this option is available.
Does FDA accept electronic signatures?
In March 1997, FDA issued final part 11 regulations that provide criteria for acceptance by FDA, under certain circumstances, of electronic records, electronic signatures, and handwritten signatures executed to electronic records as equivalent to paper records and handwritten signatures executed on paper.
Which regulation is applicable for maintaining electronic records and electronic signatures?
In March 1997, FDA issued final part 11 regulations that provide criteria for acceptance by FDA, under certain circumstances, of electronic records, electronic signatures, and handwritten signatures executed to electronic records as equivalent to paper records and handwritten signatures executed on paper.
What is electronic records and electronic signatures?
electronic records and signatures document that will have a profound effect on device companies. This rule (21 CFR 11) establishes the criteria under which FDA will deem electronic records and electronic signatures equivalent to paper records and traditional handwritten signatures.
What is FDA 21 CFR Part 11 compliance?
FDA 21 CFR Part 11 compliance dictates that those companies who use electronic systems for document and signature control must provide assurance that the electronic documents are authentic. ... FDA 21 CFR Part 11 compliance dictates that signatures whether electronic or handwritten be linked to their respective records.
Does 21 CFR part 11 apply to medical devices?
Medical device companies that wish to sell their devices in the US and EU must implement a quality management system that meets the requirements of 21 CFR Part 820 and ISO 13485:2016. ... Specifically, 21 CFR Part 11, the FDA's regulations for electronic documentation and electronic signatures.
Can a 1572 be electronically signed?
The 1572 on FDA's website may be completed by typing the information directly into the fillable form and printing the completed form. ... The completed form must be signed and dated by the investigator (either by hand or using an acceptable electronic method).
What is the FDA form 1572?
The 1572 has two purposes: 1) to provide the sponsor with information about the investigator's qualifications and the clinical site that will enable the sponsor to establish and document that the investigator is qualified and the site is an appropriate location at which to conduct the clinical investigation, and 2) to ...
What is a flattened digital signature?
Flattened digital signatures. A Flattened signature occurs when flattening pushes the appearance of the signature annotation into the page content, effectively deleting interactive elements, but retaining the visual appearance of those elements.
Ready to try pdfFiller's? Lock Up Signature Accreditation
Upload a document and create your digital autograph now.