Modify Table in the Clinical Trial Agreement Template with ease For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
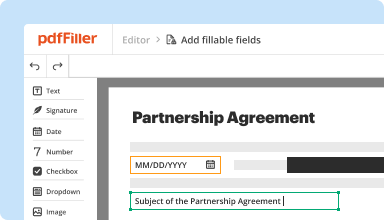
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
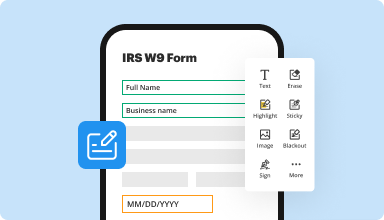
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
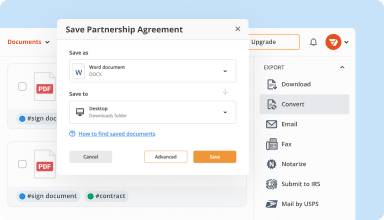
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
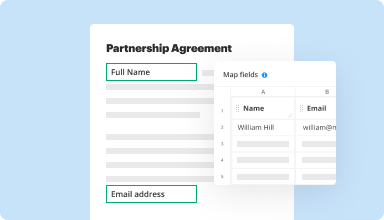
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
I like the program. I did pay for it, so I feel that I should be able to upload pictures to the file. Uploading images should be included in a basic membership. I still like the service.
2017-11-15
I hate having to type in a verification code when I leave for a bit. I know it is fro security reasons, but I would really appreciate it if you did not make it type the code.
2018-07-03
I've been using PDFfiller regularly for over 2 years now, and it works wonderfully. I'm able to upload documents, add fillable lines, and get electronic signatures seamlessly. Customer service is also great - prompt and friendly in attending to any questions or issues. Makes running my business that much easier.
2022-05-05
It is really good
It is really good, I hate forms, who doesn't but this made the chances of my cat getting kicked much smaller.
No animals were hurt in the making of this review.
2021-12-07
I have used it a few times thus far it to complete on line forms. I find it easy to use and navigate. Helpful tool to avoid unnecessary printing, writing, scanning, storing.
2021-03-21
I have got to say during this time of a…
I have got to say during this time of a COVID-19 crisis, it's nice to have a service like this where I can just get the pdf's filled out and signed. It also makes for the reader on the other end to see it more clearly. I am an extremely happy customer and will continue to be for as long as COVID is here anyway.
2020-11-13
I am a relatively new user to pdffiller, but have found the platform user friendly and does exactly what I need it to. Helping me modernise a lot of statutory requirements for e-signatures and template creations.
When I experienced issues loading documents due to permissions through my work network, the support team at pdffiller responded quickly and continued assisting myself and co-ordinated with my employers IT support function to resolve it within 24 hours, allowing me to get on with my job.
2020-09-25
A good pdf editing platform
After reading the reply from pdf filler I have decided to change my initial rating,Thanks for responding and clearing the problem up
2020-09-24
Having better results with the site It is a lifesaver as far as my clients being able to sign consents on the screen and get them back to me. So far, the recipients of my docs have been able to follow the instructions and sign the docs without difficulty
2020-04-29
Modify Table in Clinical Trial Agreement Template
The Modify Table feature in the Clinical Trial Agreement Template empowers you to easily adjust key elements in your agreement. With this tool, you maintain control over your trial setup and ensure accuracy throughout the process.
Key Features
User-friendly interface for quick modifications
Real-time updates to ensure agreement accuracy
Customizable fields to meet specific trial needs
Version tracking to maintain changes history
Secure access for team collaboration
Potential Use Cases and Benefits
Adapt agreements to changing trial conditions
Facilitate stakeholder discussions with clear modifications
Reduce errors with streamlined editing processes
Enhance compliance with updated protocols
Save time on agreement reviews through timely adjustments
This feature addresses your challenges by allowing you to make necessary changes efficiently. Whether you need to update timelines, modify participant details, or adjust funding allocations, the Modify Table feature simplifies these tasks. You can focus on what matters most: conducting a successful clinical trial.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What information should be included in a clinical trial protocol?
ing to the ICH Good Clinical Practice guidelines, a protocol should include the following topics: Title Page (General Information) Background Information. Objectives/Purpose. Study Design. Selection and Exclusion of Subjects. Treatment of Subjects. Assessment of Efficacy. Assessment of Safety.
What are the components of a clinical trial agreement?
Clinical Trial Agreements typically involve a trial sponsor (a pharmaceutical, biotech, or medical device company), a research institution and a principal investigator. Separate agreements are negotiated in their entirety for each clinical trial.
What is a clinical trial amendment?
Amendments are changes made to a clinical trial after it has received regulatory approval. An amendment can take a significant amount of time and resources to develop, review and implement at participating sites. This can affect the efficient delivery of clinical trials and potentially contribute to research waste.
What is the content of CRF in clinical trials?
Most commonly used standard CRF templates are inclusion criteria, exclusion criteria, demography, medical history, PE, AE, concomitant medication and study outcome modules, whereas, the modules which captures efficacy data are not unique. Their design varies from study to study depending on the protocol specifications.
What is included in a clinical trial agreement?
A clinical trial agreement (CTA) or clinical study agreement (CSA) is a legally binding agreement that governs the conduct of a particular study and sets forth the obligations of each party to the agreement.
What are the basic components of an agreement?
Whether a contract is 200 pages or 10 pages, to be a legally binding agreement they must contain six basic elements: Offer, Acceptance, Awareness, Consideration, Capacity, Legality.
What is a Phase 1 trial?
The first step in testing a new treatment in humans. A phase I clinical trial tests the safety, side effects, best dose, and timing of a new treatment. It may also test the best way to give a new treatment (for example, by mouth, infusion into a vein, or injection) and how the treatment affects the body.
What is an accelerated clinical trial agreement?
The University of Michigan (along with other industry partners and institutions) uses the Accelerated Clinical Trial Agreement (ACTA). For those industry partners who agree to use the agreement (and the sponsor must agree to use it), it decreases contract negotiation time.
#1 usability according to G2
Try the PDF solution that respects your time.






