Order Equation Record For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
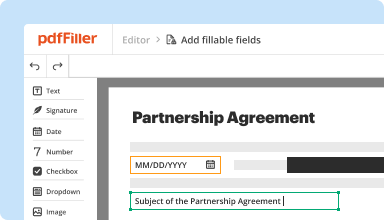
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
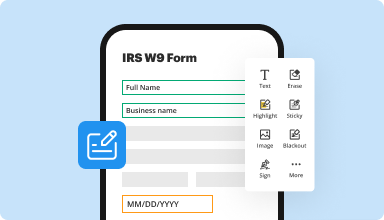
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
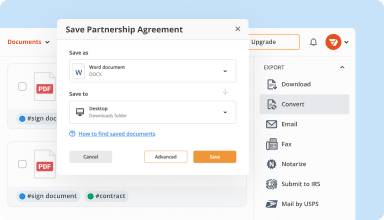
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
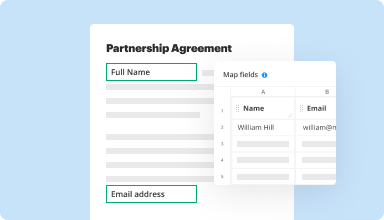
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
I was gonna select only 4 stars since I was having difficulty getting the app to do all that it claims, but since customer service was so great I had to add the additional star.
2015-06-30
I was having difficulty finding the legal documents I needed fillable copies you provided the access I needed. and my software wasn't allowing me to just fill in the documents online, I am finding my way around but if I can make it easier I am all in.
2016-02-25
The tasks I needed to complete I was able to facilitate with PDFiller. I was later informed of a more economical manner. I manage a non-profit org. We always need to be conscious of cost.
2016-06-10
I worked with Elie tonight and your support is amazing. She was friendly, patient and knowledgeable. She was able me to better understand the PDFfiller and was able to accomplish what I wanted. Very Pleased with your service and product.
2017-01-27
Extremely Useful, Slightly Non-Intuitive
Love the program/service/software and all its online functions, including the US Legal Forms integration for documents like wills and trusts. But it is a little clunky/non-intuitive in terms of user experience and interaction–be prepared to spend time clicking around to find not only your own documents, but also the service's libraries. Looking forward to integrating with third-party/external cloud storage.
2024-01-19
Highly Satisfied
I found the app simple to use. I did not expect such fast human responses to queries. Impressed with the customer service and support.
2023-07-22
As good as DocuSign
Allows you to create pdfs that with blanks in order to easily fill out.
There are no free versions, so you pretty much just hope that a business has taken the proper steps to acquire this and utilize it.
2021-04-09
The product is great and very easy to…
The product is great and very easy to use. I had started a free trial for one time use, but forgot to cancel it and was charged for a full year, but when I contacted them they refunded my money straight away with no questions asked. I wish all companies were as good to deal with as this!
2021-03-04
I love the features and ease of use of…
I love the features and ease of use of this service. It really has been a great asset to my Resume Service. Thank you so much! Kind Regards, Lucinda Kerrigan, CPRW
2021-01-21
Order Equation Record Feature
The Order Equation Record feature streamlines the way you handle orders. It simplifies tracking, managing, and recording orders for your business. With this tool, you gain clarity and efficiency, which leads to better decision-making and customer satisfaction.
Key Features
Centralized order tracking system
User-friendly interface for easy navigation
Real-time updates on order status
Automated notifications for order milestones
Customizable reporting options
Potential Use Cases and Benefits
Retail businesses can manage customer orders more efficiently
E-commerce platforms can offer better order visibility to customers
Service industries can streamline client requests and confirmations
Logistics companies can enhance delivery tracking and communication
Small businesses can save time and reduce errors in order management
This feature addresses common challenges in order management. By providing a straightforward and organized approach, it helps you reduce lost orders and delays. When you implement the Order Equation Record feature, you create a smoother workflow that enhances customer trust and satisfaction.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do you determine the order of a reaction experimentally?
Methods of Determining Reaction Order. Either the differential rate law or the integrated rate law can be used to determine the reaction order from experimental data. Often, the exponents in the rate law are the positive integers: 1 and 2 or even 0. Thus, the reactions are zeroth, first, or second order in each reactant ...
How do you determine the order of a reaction from data?
Compare the data from two experiments to determine the effect on the reaction rate of changing the concentration of a species.
Compare the observed effect with behaviors characteristic of zeroth- and first-order reactions to determine the reaction order. Write the rate law for the reaction.
How do you determine reaction order?
The overall order of the reaction is found by adding up the individual orders. For example, if the reaction is first order with respect to both A and B (a = 1 and b = 1), the overall order is 2. We call this an overall second order reaction.
How do you determine the order of a reaction from a graph?
First order, would be natural log of concentration A versus time. If you get a straight line with a negative slope, then that would be first order. For second order, if you graph the inverse of the concentration A versus time, you get a positive straight line with a positive slope, then you know it's second order.
What is the order with respect to each reactant?
The overall reaction order is the sum of the orders with respect to each reactant. If m = 1 and n = 1, the overall order of the reaction is second order (m + n = 1 + 1 = 2). The rate law: describes a reaction that is first order in hydrogen peroxide and first order overall.
How do you determine first order reaction?
A first-order reaction depends on the concentration of one reactant, and the rate law is: r=dad=k[A] r = DA DT = k [A] .
R=d[A]DT=k[A]
2N2O5(g)4NO2(g)+O2(g)
Rate=k[N2O5]m.
Rate=k[N2O5]1=k[N2O5]
1.4×103=k(0.020)
k=0.070s1.
How do you determine rate law?
Remember, rate laws, or rate equations, relate the concentrations of reactants with the speed of the reaction. You can determine x and y in the rate law by looking at experimental data and noticing how the change in concentration of a reactant is related to the reaction rate.
How do you determine the order of a reaction from experimental data?
Compare the data from two experiments to determine the effect on the reaction rate of changing the concentration of a species.
Compare the observed effect with behaviors characteristic of zeroth- and first-order reactions to determine the reaction order. Write the rate law for the reaction.
How do you determine the order of a reaction example?
Add the exponents of each reactant to find the overall reaction order. This number is usually less than or equal to two. For example, if reactant one is first order (an exponent of 1) and reactant two is first order (an exponent of 1) then the overall reaction would be a second order reaction.
#1 usability according to G2
Try the PDF solution that respects your time.






