Protected Table Of Contents Accreditation For Free
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

Upload a document

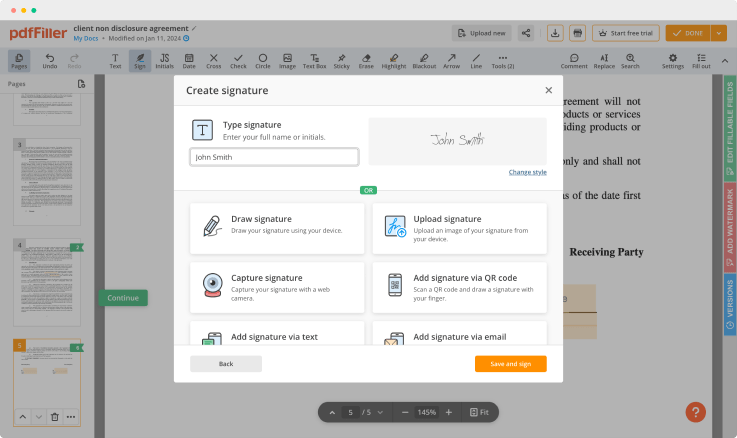
Generate your customized signature

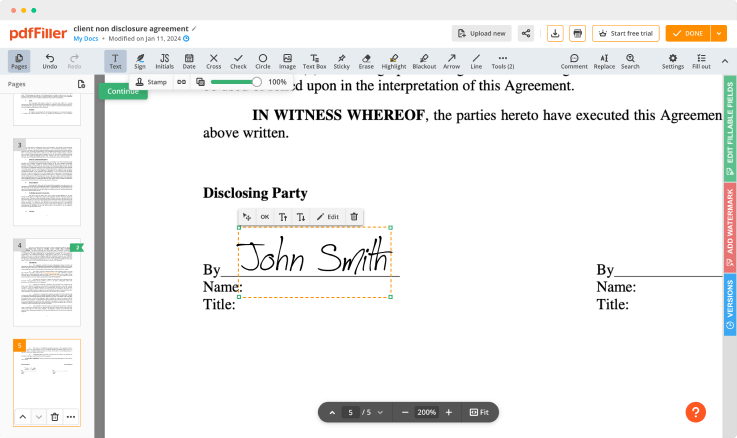
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
How to Add a Signature to PDF (and Send it Out for Signature)
Watch the video guide to learn more about pdfFiller's online Signature feature

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Protected Table Of Contents Accreditation Feature
The Protected Table Of Contents Accreditation feature enhances document security and organization. This tool helps you manage and protect the integrity of your documents while providing easy access to essential sections. With this feature, you can confidently share your content, knowing that it remains secure and well-structured.
Key Features
Secure access to document sections
Easy navigation through the table of contents
Customizable section protection settings
Clear marking of accredited content
User-friendly interface for quick adjustments
Potential Use Cases and Benefits
Ideal for educational institutions ensuring students access accredited content
Useful for businesses requiring strict control over corporate documents
Supports compliance with regulatory standards for documentation
Enhances readability for users by providing a clear navigation path
Facilitates collaboration by defining access levels for different users
This feature addresses the common problem of document mismanagement. By securing your table of contents, you protect valuable information from unauthorized changes and enhance user experience with seamless navigation. You can rest assured that your essential content is accessible yet secure.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How long is CAP accreditation good for?
A laboratory inspection occurs every two years. In the years when an on-site inspection does not occur, the laboratory must perform a self- inspection using materials provided by the CAP. The accreditation program is voluntary and helps laboratories achieve the highest standards of excellence in patient care.
What does it mean to be CAP accredited?
CAP stands for College of American Pathologists. This certification is a peer-based inspection process that combines regulatory and educational coaching from the world's most respected pathology organization. Certification helps lab professionals: Reach the highest standard of lab quality.
What is the difference between CAP and CIA?
CIA is the set of federal laws/regulations that almost every lab in the USA must follow. CAP is an accrediting agency that has rules that are stricter than CIA. State regulations (if they exist) are generally somewhere in between.
What does Cap mean in laboratory?
CAP stands for College of American Pathologists. This certification is a peer-based inspection process that combines regulatory and educational coaching from the world's most respected pathology organization. Certification helps lab professionals: Reach the highest standard of lab quality.
How often cap lab inspect?
On-site inspections occur every two years using CAP Accreditation Checklists to assess compliance with program requirements.
How do I prepare for a cap inspection?
Know Your Checklists. One of the most important steps in preparing for a CAP inspection is to make sure you are using the correct version of the CAP checklists. Revisit the Past. Organize Stuff and Staff. Remain Calm. Be Present and Accountable.
What is a cap Phase II deficiency?
Phase I deficiencies require a written response indicating corrective action taken. Phase II deficiencies require a written response and supporting documentation demonstrating compliance. The response should explain the purpose of the documentation submitted.
What is the difference between CIA and CAP?
In simple terms, being CIA certified and CAP accredited ensures your test results are meeting and exceeding industry standards for clinical laboratory testing. The College of American Pathologists (CAP) is such an agency. The CAP releases its own requirements building upon CIA '88 regulations.
Ready to try pdfFiller's? Protected Table Of Contents Accreditation
Upload a document and create your digital autograph now.