Regulate Approve Application For Free
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

Upload a document

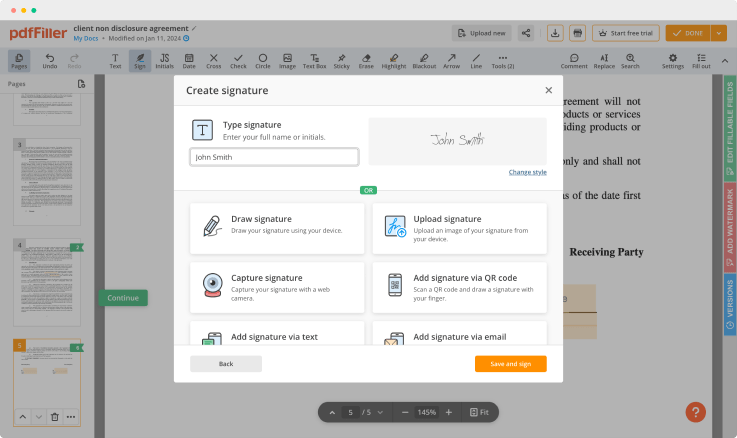
Generate your customized signature

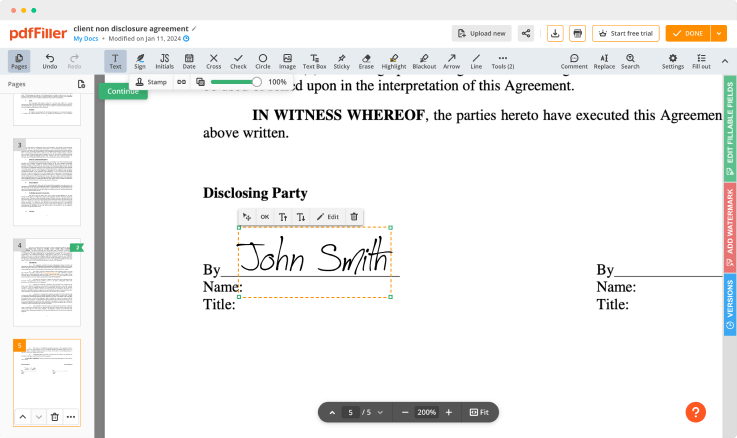
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
How to Add a Signature to PDF (and Send it Out for Signature)
Watch the video guide to learn more about pdfFiller's online Signature feature

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Regulate Approve Application Feature
The Regulate Approve Application feature simplifies the process of managing approvals within your organization. It streamlines workflows, ensuring that you maintain compliance while efficiently handling requests. With this feature, you can transform the way you oversee approvals, making it easier for you to focus on what truly matters.
Key Features
User-friendly interface for easy navigation
Customizable approval workflows to suit your needs
Real-time notifications to keep you informed
Robust reporting tools for tracking and analysis
Secure access control to protect sensitive information
Potential Use Cases and Benefits
Streamlining approval processes to enhance productivity
Reducing approval times for faster decision-making
Ensuring compliance with industry regulations
Improving communication among team members
Facilitating better resource allocation
By using the Regulate Approve Application feature, you can solve common issues related to approval delays and compliance risks. This feature helps you gain control over the approval process, enabling you to minimize errors and reduce bottlenecks. Ultimately, you can create a more efficient workflow that supports your strategic goals.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What does CDR regulate?
As part of the U.S. Food and Drug Administration (FDA), CDR regulates over-the-counter and prescription drugs, including biological therapeutics and generic drugs. This work covers more than just medicines. For example, fluoride toothpaste, antiperspirants, dandruff shampoos and sunscreens are all considered “drugs.”
What is the difference between CDR and CBR?
When a product's primary mode of action is attributable to a type of biological product assigned to CDR, the product will be assigned to CDR. Similarly, when a product's primary mode of action is attributable to a type of biological product assigned to CBR, the product will be assigned to CBR.
What does CDR stand for?
The Center for Drug Evaluation and Research (CDR, pronounced “see'Oder”) is a division of the U.S. Food and Drug Administration (FDA) that monitors most drugs as defined in the Food, Drug, and Cosmetic Act.
What is CBR FDA?
CBR is the Center within FDA that regulates biological products for human use under applicable federal laws, including the Public Health Service Act and the Federal Food, Drug and Cosmetic Act.
What is the difference between BLA and NDA?
Whereas a new drug application (NDA) is used for drugs subject to the drug approval provisions of the FDC Act, a biologics license application (BLA) is required for biological products subject to licensure under the PHS Act. FDA approval to market a biologic is granted by issuance of a biologics license.
How does the FDA regulate pharmaceutical companies?
Regulating new drugs The FDA only approves drugs if they are deemed both safe and effective for their intended purpose. The manufacturing plant that produces the drug must pass a rigorous safety inspection and the drug must be labeled correctly, meaning it is prescribed for the conditions it was intended to treat.
Does the FDA regulate prescription drugs?
The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation
What does the FDA do to regulate the drug industry?
The U.S. Food and Drug Administration's original purpose was to regulate the selling of disbanded food and drugs. The U.S. Food and Drug Administration (FDA) is the government agency responsible for reviewing, approving and regulating medical products, including pharmaceutical drugs and medical devices.
Ready to try pdfFiller's? Regulate Approve Application
Upload a document and create your digital autograph now.