Regulate Approve Format For Free
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

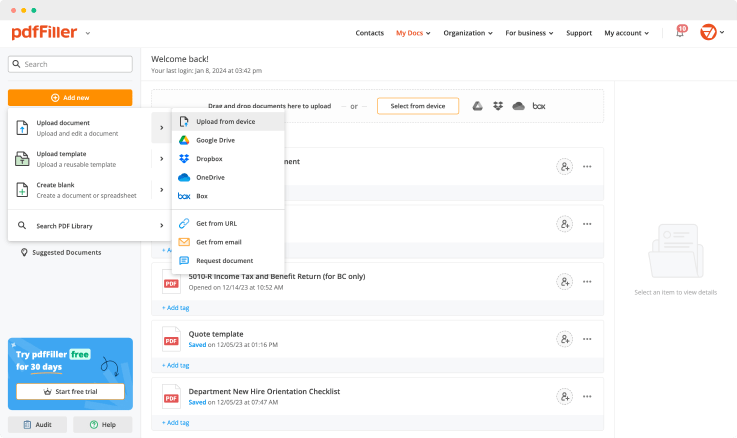
Upload a document

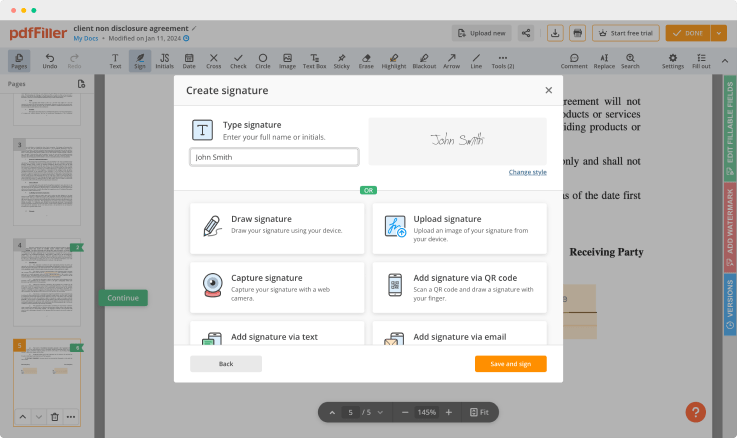
Generate your customized signature

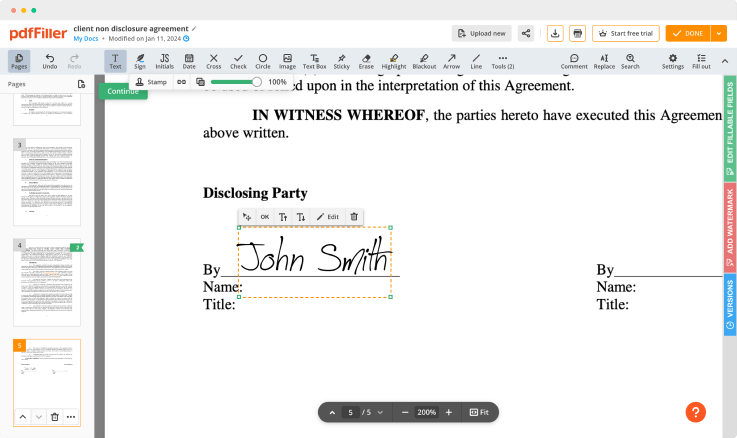
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
How to Add a Signature to PDF (and Send it Out for Signature)
Watch the video guide to learn more about pdfFiller's online Signature feature

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Regulate Approve Format Feature
The Regulate Approve Format feature streamlines your document approval process, ensuring compliance and efficiency. It transforms how organizations manage their formatting and approvals, making it easier for you to achieve your goals.
Key Features
Standardizes document formats for consistency
Facilitates quick approvals with automated workflows
Tracks changes and revisions for transparency
Integrates with existing tools you already use
Offers customizable templates for various needs
Potential Use Cases and Benefits
Enhance collaboration across teams during document creation
Speed up the review process to meet deadlines
Reduce errors and miscommunication in document submissions
Streamline compliance checks for regulatory bodies
Improve overall productivity by automating routine tasks
By implementing the Regulate Approve Format feature, you address common challenges in document management. It minimizes delays, reduces confusion, and fosters a focused environment for your team. You'll not only see an increase in efficiency but also a boost in the quality of your outputs.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do I submit an FDA approval?
To get FDA approval, drug manufacturers must conduct lab, animal, and human clinical testing and submit their data to FDA. FDA will then review the data and may approve the drug if the agency determines that the benefits of the drug outweigh the risks for the intended use.
How do I submit to the FDA?
FDA's preferred method of submission is via the FDA Electronic Submissions Gateway (ESG). For more information, see the Electronic Submissions Gateway web page. For automated processing of your submissions, use the ESG and submit an FDA fillable form with each submission.
How do I submit documents to the FDA?
Upload the documents into Import Trade Auxiliary Communication System (MITACS). Use the find an import office contact page to determine your local import division email address, postal address, and/or FAX number to submit documents outside MITACS.
How do I submit ECTD to FDA?
Learn About ECTD. Review the Electronic Submission Resources. Submit Fillable Forms and Compliant PDFs. Request an Application Number. Register for an Electronic Submissions Gateway Account. Send a Sample Submission to FDA. Submit Via the Electronic Submission Gateway.
What is an FDA submission?
The Food and Drug Administration (FDA) Electronic Submissions Gateway (ESG) is an Agency-wide solution for accepting electronic regulatory submissions. The FDA ESG enables the secure submission of premarket and postmarked regulatory information for review.
What are the FDA guidelines?
Guidance documents represent FDA's current thinking on a topic. They do not create or confer any rights for or on any person and do not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations.
Does my product need FDA approval?
Products requiring FDA premarket approval: According to the FDA, the product's benefits must outweigh any risks related to its intended use. Animal drugs and food additives in animal food, which includes pets, poultry, and livestock. Medical devices. Approval depends on the risk classification of the device.
Can products be sold without FDA approval?
In some cases, FDA's enforcement efforts focus on products after they are already for sale. That is determined by Congress in establishing FDA's authorities. Even when FDA approval is not required before a product is sold, the agency has regulatory authority to act when safety issues arise.
Ready to try pdfFiller's? Regulate Approve Format
Upload a document and create your digital autograph now.