Regulate Bookmark Diploma For Free




Join the world’s largest companies









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Regulate Bookmark Diploma Feature
Discover the Regulate Bookmark Diploma feature, designed to enhance your digital organization and streamline your academic pursuits. With this feature, you can manage essential bookmarks efficiently, ensuring you never lose track of your important documents and resources.
Key Features
Potential Use Cases and Benefits
The Regulate Bookmark Diploma feature solves the problem of information overload. It provides a systematic way to keep your academic materials organized, allowing you to focus more on learning and less on searching. By enabling quick access to your essential resources, you can boost your efficiency and confidence in managing your studies.
Instructions and Help about Regulate Bookmark Diploma For Free
Regulate Bookmark Diploma: easy document editing
Document editing has turned into a routine procedure for the people familiar to business paperwork. It is easy to adjust almost every Word or PDF file, using numerous software and tools that allow applying changes to documents. On the other hand, such apps take up space while reducing its battery life drastically. Online PDF editing tools are much more convenient for most users, though the vast part don't cover all the needs.
Now there is a right tool to edit PDF files and much more, online and easily.
pdfFiller is an all-in-one solution that allows you to save, produce, modify and sign your documents online. It supports not only PDFs but other common file formats, such as Word, JPG and PNG images, PowerPoint and more. Upload documents from your device and start editing in just one click, or create a new one yourself. All you need to start processing PDFs online with pdfFiller is any internet-connected device.
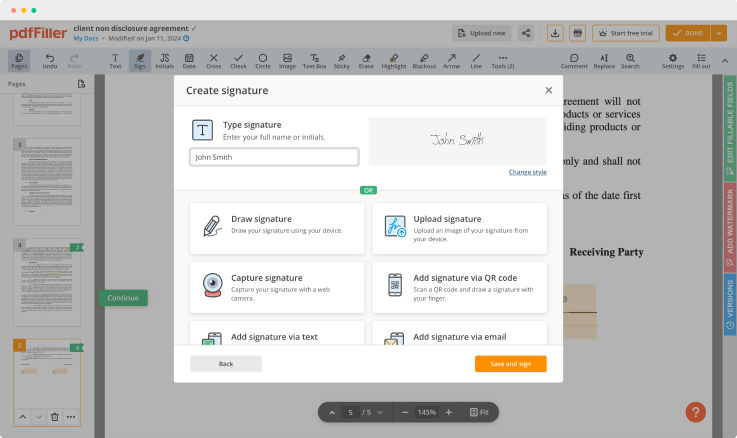
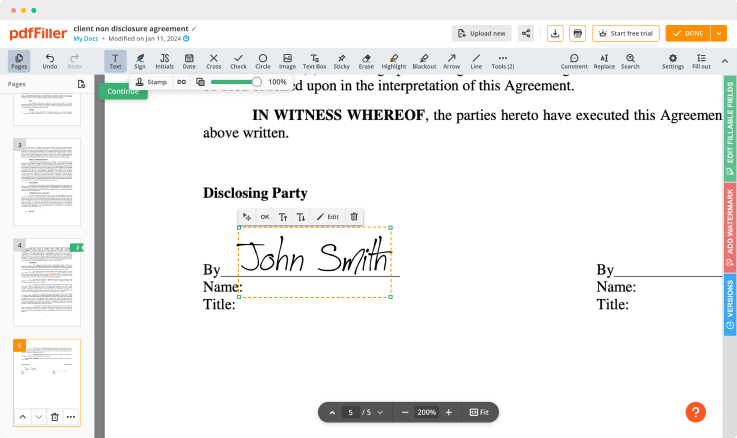
pdfFiller comes with a multi-purpose text editing tool, so you can rewrite the content of your document efficiently. A great range of features makes it possible to change not only the content but the layout, to make your documents look professional. Furthermore, the pdfFiller editing tool enables you to edit pages in your form, put fillable fields, add images and graphic elements, change text spacing and alignment, and so on.
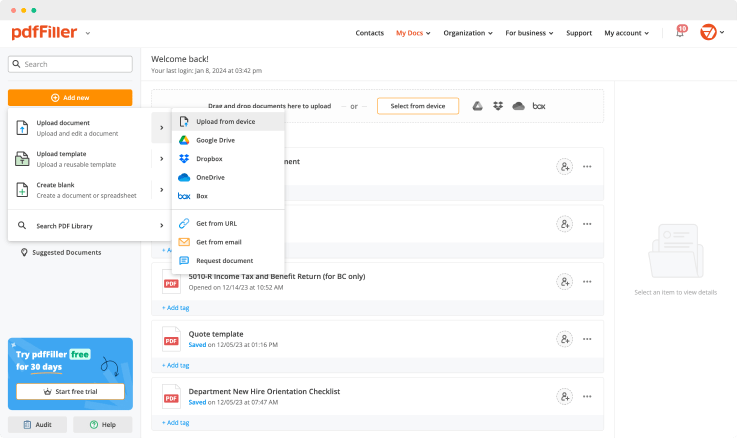
Use one of these methods to upload your document template and start editing:
Once uploaded, all your documents are easily available from the Docs folder. All your templates will be securely stored on a remote server and protected with advanced encryption. It means they cannot be lost or opened by anyone else but yourself and users you share your document with. Save time by quickly managing documents online using just your web browser.
For pdfFiller’s FAQs
Ready to try pdfFiller's? Regulate Bookmark Diploma