Regulate Day Letter For Free
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

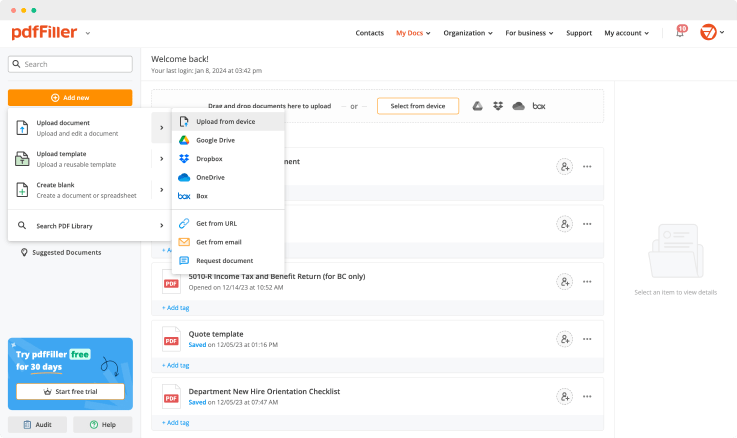
Upload a document

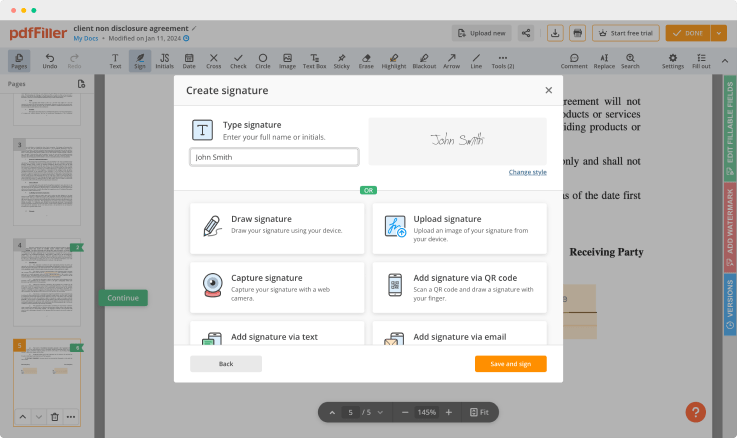
Generate your customized signature

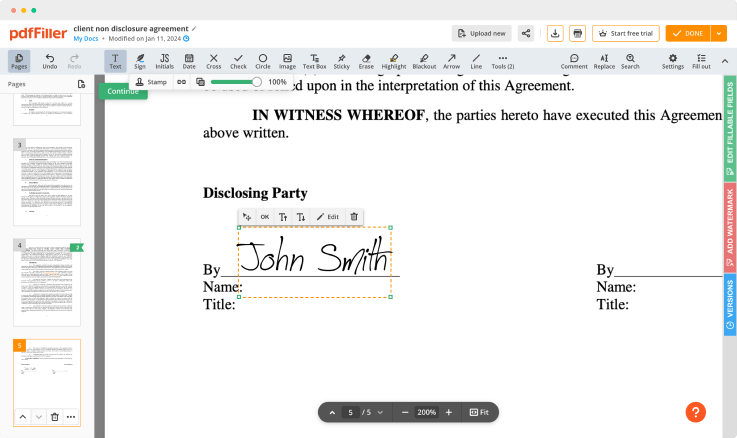
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
Video Review on How to Regulate Day Letter

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Regulate Day Letter Feature
Meet the Regulate Day Letter feature, your essential tool for efficient communication and organization. This feature simplifies your daily planning and ensures you stay on track with your tasks and appointments.
Key Features
Customizable templates for various occasions
User-friendly interface for easy navigation
Automated reminders for upcoming deadlines
Integration with popular calendars and apps
Support for multiple languages to reach a wider audience
Potential Use Cases and Benefits
Plan and manage daily activities effectively
Communicate important updates and messages clearly
Aid in team collaboration with shared letters
Help parents organize school schedules and activities
Assist professionals in managing client communications
The Regulate Day Letter feature addresses your need for clarity and control in your daily tasks. By providing customizable templates and timely reminders, it eliminates confusion and missed deadlines. With this feature, you can streamline your communication and ensure you never miss an important moment.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What happens after an FDA warning letter?
What is a Warning Letter? After a Form 483 is issued and the inspector completes the Establishment Inspection Report, the agency may issue an FDA Warning Letter. A warning letter indicates that higher FDA officials have reviewed the observations and that a serious violation may exist.
How do you respond to an FDA warning letter?
Step 1: Establish a Timeline for Response Activities. Step 2: Identify Root Cause. Step 3: Issuing Caps. Step 4: Establish a Timeline for Addressing 483s. Step 5: Draft Initial Response Letter. Step 6: Consistent Follow Up.
How do you respond to FDA 483?
Step 1: Establish a Timeline for Response Activities. Step 2: Identify Root Cause. Step 3: Issuing Caps. Step 4: Establish a Timeline for Addressing 483s. Step 5: Draft Initial Response Letter. Step 6: Consistent Follow Up.
How do you respond to a warning letter?
Compose yourself before you respond. You are tensed because this is a sign that you might be jobless soon. Avoid being defensive. Respond in writing. Keep the matter private and act with respect. Try and fix your mistakes. Get back into the job market.
What happens after FDA warning letter?
What is a Warning Letter? After a Form 483 is issued and the inspector completes the Establishment Inspection Report, the agency may issue an FDA Warning Letter. A warning letter indicates that higher FDA officials have reviewed the observations and that a serious violation may exist.
What does an FDA warning letter mean?
An FDA warning letter is an official message from the United States Food and Drug Administration (FDA) to a manufacturer or other organization that has violated some rule in a federally regulated activity. A Warning Letter is one of the Agency's principal means of achieving prompt voluntary compliance with the Act.
Is a 483 a warning letter?
An FDA 483 observation is a notice that highlights potential regulatory problems, while a warning letter is an escalation of this notice. You need to respond in writing within 15 days of receiving both a 483 and a warning letter.
What is the difference between FDA Form 483 and Warning Letter?
The Form 483 is issued by the inspection team alone. The warning letter is issued from a higher level FDA official or officials. Warning letters usually result from multiple lacking responses to issued 483s, or other issues much more serious that require quick attention/escalation.
Ready to try pdfFiller's? Regulate Day Letter
Upload a document and create your digital autograph now.