Regulate Initials Paper For Free




Join the world’s largest companies









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Regulate Initials Paper Feature
The Regulate Initials Paper feature helps you manage and streamline your document processes effortlessly. This unique product allows you to keep track of initials and signatures while enhancing organization and efficiency.
Key Features
Potential Use Cases and Benefits
In summary, Regulate Initials Paper addresses your document management challenges by simplifying the tracking of initials and signatures. You can increase your productivity and ensure a smoother workflow with this reliable tool. Experience peace of mind as you manage your documents with confidence.
Instructions and Help about Regulate Initials Paper For Free
Regulate Initials Paper: make editing documents online a breeze
Document editing is a routine process for the people familiar to business paperwork. You're able to modify a PDF or Word file efficiently, using a range of software and tools which allow applying changes to documents in one way or another. Nevertheless, these solutions are applications and require some space on your device and affect its performance. Processing PDF templates online, on the other hand, helps keep your computer running at optimal performance.
Luckily, you now have the option of avoiding all of these complications working with templates online.
pdfFiller is an all-in-one solution that allows to save, produce, edit your documents online. It supports primary document formats, e.g., PDF, Word, PowerPoint, JPEG, PNG and text. Create new document from scratch or upload it from your device in literally one click. All you need to start editing is an internet-connected device.
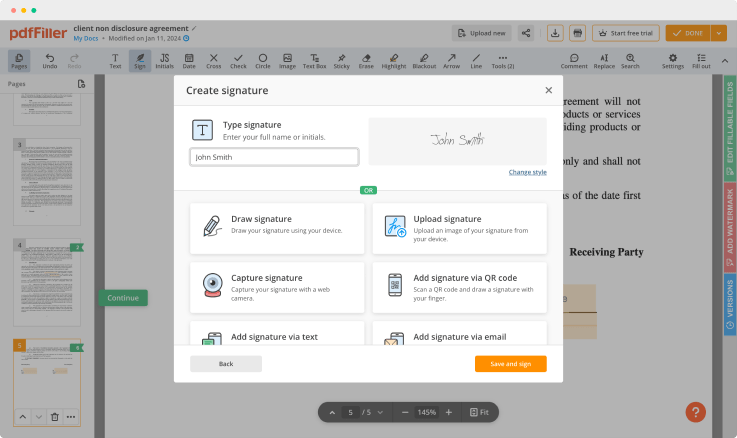
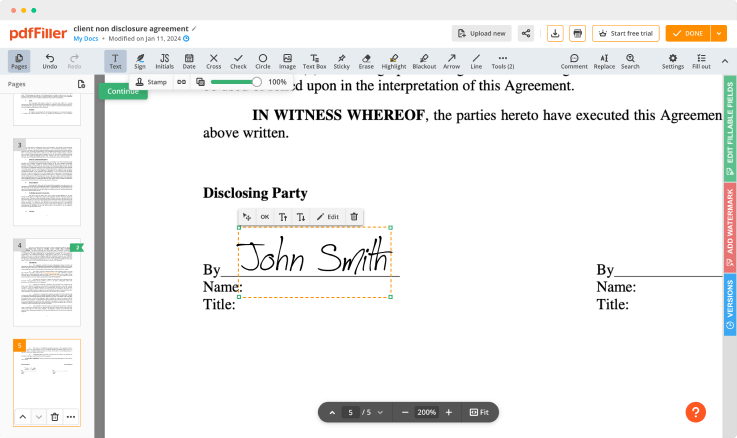
pdfFiller is equipped with an all-in-one online text editor to simplify the online process for all users. It features a variety of tools you can use to modify your form's layout making it look professional. Edit pages, place fillable fields anywhere on the form, add spreadsheets and images, change the text formatting and put a signature — it's all in one editor.
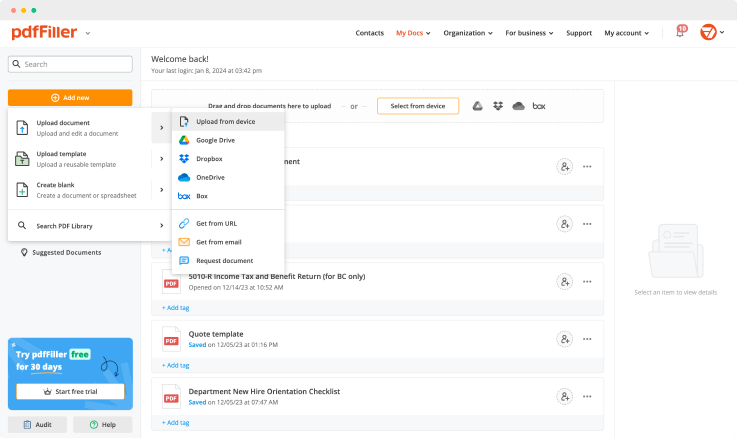
Create a document yourself or upload an existing one using the next methods:
Access every document you worked with by simply browsing to the Docs folder. pdfFiller stores all your data encrypted and on remote server, to provide you with extra level of security. Your information is accessible across all your devices immediately, and you're in control of who can work with your documents. Move all your paperwork online and save time.
For pdfFiller’s FAQs
Ready to try pdfFiller's? Regulate Initials Paper