Regulate Payment Diploma For Free




Join the world’s largest companies









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Regulate Payment Diploma
The Regulate Payment Diploma offers a robust framework for understanding and managing payment regulations effectively. This program equips you with the knowledge to navigate complex payment environments.
Key Features
Potential Use Cases and Benefits
By enrolling in the Regulate Payment Diploma, you can tackle the challenges of understanding payment regulations head-on. This program aids in minimizing compliance risks, improving transaction security, and ensuring your organization meets regulatory standards. With a clear grasp of the legal landscape, you can make better decisions and contribute to your team's success.
Instructions and Help about Regulate Payment Diploma For Free
Regulate Payment Diploma: edit PDF documents from anywhere
When moving a document management online, it's essential to get the PDF editor that meets your needs.
In case you hadn't used PDF for your documents before, you can switch anytime — it's simple to convert any other format into PDF. This makes creating and using most of them simple. You can also create just one PDF to replace multiple documents of different formats. That’s why the Portable Document Format ideal for basic presentations and reports.
Though many solutions allows PDF editing, it’s hard to find one that covers all PDF editing features available, at a reasonable price.
With pdfFiller, you are able to annotate, edit, convert PDFs into other formats, fill them out and add an e-signature in the same browser tab. You don’t have to install any programs. It’s an extensive platform you can use from any device with an internet connection.
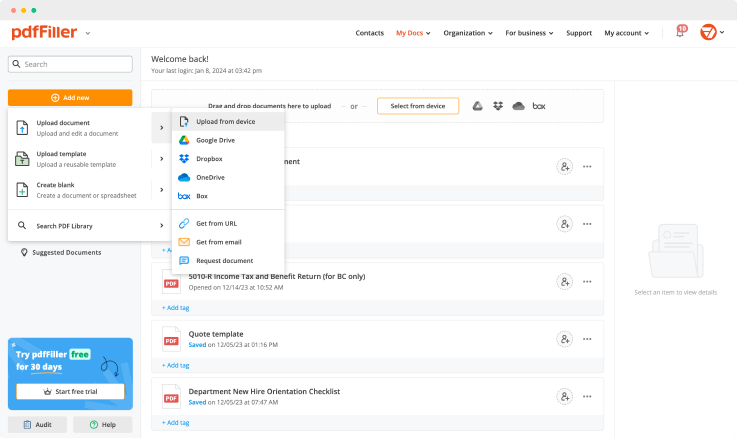
Create a document yourself or upload a form using the following methods:
Once you uploaded the document, it’s saved and can be found in the “My Documents” folder.
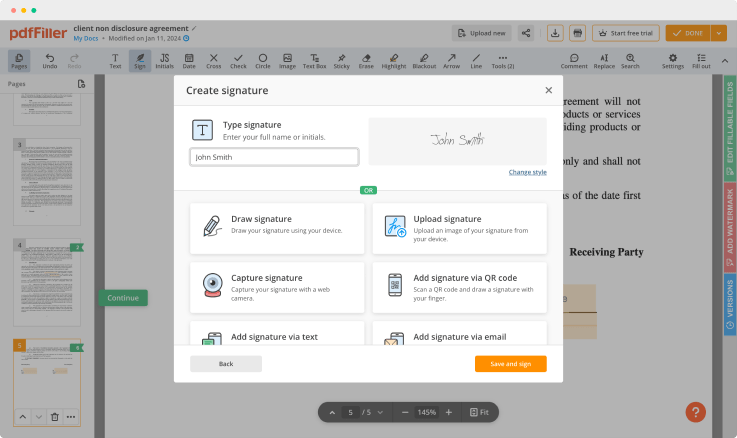
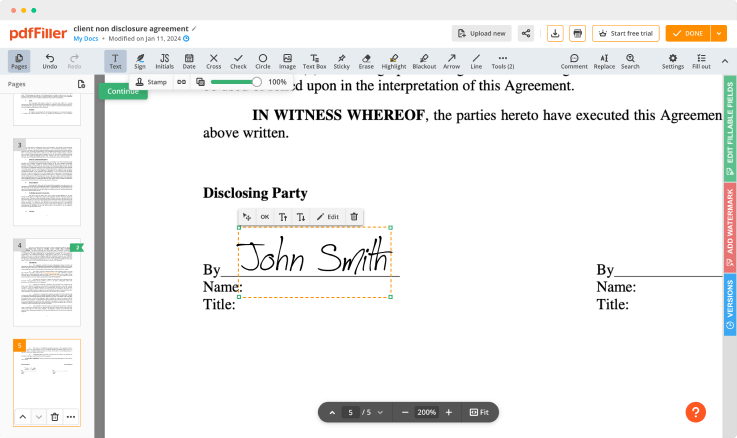
Use powerful editing tools such as typing text, annotating, and highlighting. Change a document’s page order. Once a document is completed, download it to your device or save it to the third-party integration cloud. Collaborate with users to fill out the fields and request an attachment. Add images to your PDF and edit its layout. Add fillable fields and send to sign.
For pdfFiller’s FAQs
Ready to try pdfFiller's? Regulate Payment Diploma