Regulate Sum Voucher For Free
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

Upload a document

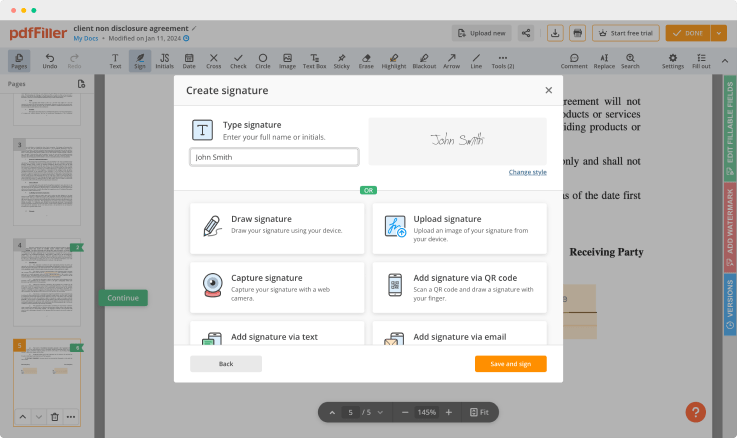
Generate your customized signature

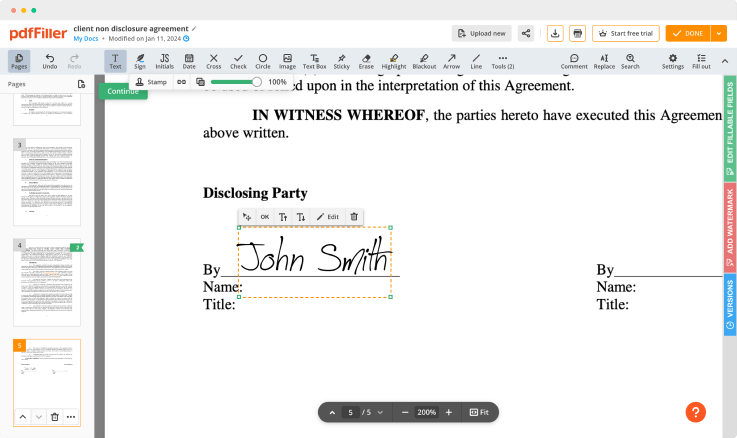
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
How to Add a Signature to PDF (and Send it Out for Signature)
Watch the video guide to learn more about pdfFiller's online Signature feature

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Regulate Sum Voucher Feature: Streamline Your Financial Management
The Regulate Sum Voucher feature offers a simple solution for effectively managing your budget. This tool helps you track, allocate, and regulate your funds with ease. You can gain control over your finances and make informed decisions comfortably.
Key Features of Regulate Sum Voucher
Easy fund tracking and allocation
Customizable budget categories
User-friendly interface for quick access
Real-time financial insights
Secure and reliable transactions
Potential Use Cases and Benefits
Personal budgeting for daily expenses
Managing business expenses more efficiently
Facilitating group budgets for events or projects
Tracking donations or grants for organizations
Enabling better expense reporting for employees
This feature can solve your financial management problems by providing clear visibility into your spending habits. With real-time tracking, you can identify areas for improvement and make smarter financial decisions. The Regulate Sum Voucher makes budgeting manageable, allowing you to focus on what truly matters.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do I get a priority review voucher?
Eligible candidates are granted two vouchers and receive priority review for each voucher: the drug winning a voucher for a neglected or rare disease, and the drug using a voucher for another indication. By moving a drug to faster review, there is the potential to slow other drugs.
What is an FDA priority review voucher?
Priority review. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for drugs that might otherwise not be profitable to develop because of a smaller pool of patients needing treatment.
How much is a priority review voucher worth?
The highest price paid for a priority review is $350 million in August 2015, when United Therapeutics sold its voucher to Abbie. And in May 2015 Retrofit sold a PRV, originally transferred from Asclepius Pharma, to Sanofi for $245 million.
What does FDA priority review mean?
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. When seeking approval for a drug, manufacturers can apply to the FDA for priority review.
How long does FDA priority review take?
Priority Review means that the time it takes FDA to review a new drug application is reduced. The goal for completing a Priority Review is six months.
What is the FDA fast track for drug approval?
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs which treat a serious or life-threatening condition and fill an unmet medical need. Fast Track designation must be requested by the drug company.
How long does FDA fast track approval take?
Fast Track Process The drug developer must request the Fast Track designation. The request can be made at any time during the development process and the FDA will review and make a decision within sixty days.
What is the biggest difference between a New Drug Application NDA seeking standard approval versus accelerated approval?
The difference is that drugs granted accelerated approval must promptly conduct post-marketing confirmatory trials to verify the clinical benefit (as early as underway at the time the marketing application is submitted).
Ready to try pdfFiller's? Regulate Sum Voucher
Upload a document and create your digital autograph now.