Review Day Voucher For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
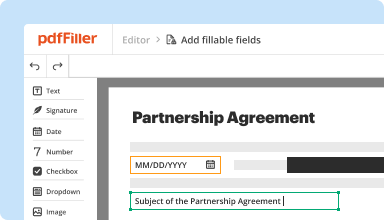
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
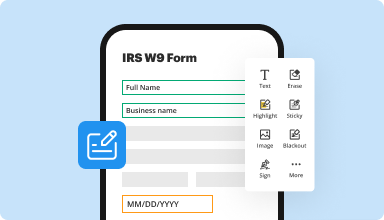
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
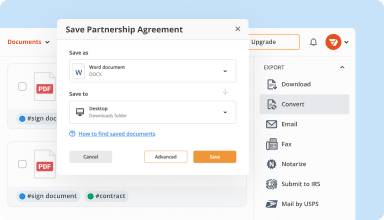
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
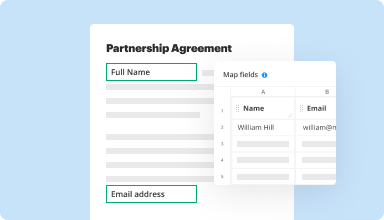
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
Unfortunately I paid for PDFescape before I found you. I subscribed to the Ultimate - it was very user unfriendly with absolutely no online tutorials or help - and I am no novice, I have designed and published several of my own websites. I really think yours is great and have had real a good experience working with setting up my contracts with it. Thanks! Sam
2017-05-16
PDF filler seems to be very useful. Is/was slightly difficult to figure out but once understood is fairly easy to use. Unfortunately the price is too high for those of us on fixed income. Is there anything lower cost for disabled seniors? Please?
2017-08-24
I am finding it very helpful. I would be interested in learning how to use it better, so a webinar would be helpful. We have a business account and most of our staff do not know how to use it at all yet, so a webinar would be really helpful.
2018-03-09
I love this program because it is so easy to use. All of my forms look very professional. After the form is completed, I can save, email or print it. I won't use another program.
2018-12-27
I am satisfied with the PDF filler. I will not need it often enough to pay a monthly fee. We will only use once or twice a year at the very most for personal use.
2019-07-29
Better and more powerful document management and editing on the web
Editing my PDFs without difficulty
This PDFfiller tool for online use is very useful since most PDF editors are paid and not all people can pay for such software. It also allows you to upload the documents you have in your Google Drive account, Dropbox and other tools
I do not find you disadvantaged, because you are covering the need of users who can not afford desktop software, to edit a PDF document
2018-07-08
Kara
Kara, had so much patience for me and my situation and I was annoyed with my self for how little I knew about technology, but hung in there with and walked me through it and got me where I need to be to get what I came for, She is a great asset to your organization. Thanks for hiring her!!!!
2023-09-20
I have found pdffiller far more…
I have found pdffiller far more intuitive and easy to use compared to the online Adobe applications. Not to mention far better value for money.
2021-02-27
I am very excited to use this product
I am very excited to use this product. I was able to troubleshoot much on my own. My only struggle was in saving final product and bringing back up to update.
2020-08-20
Review Day Voucher Feature
The Review Day Voucher feature is designed to enhance customer engagement and boost your brand's visibility. This tool enables businesses to reward customers who participate in review activities, driving loyalty and increasing sales potential.
Key Features
Generates vouchers automatically for customers who leave reviews
Customizable discount options to suit your business needs
User-friendly interface for seamless integration into your platform
Real-time tracking of voucher usage and customer feedback
Supports multiple review platforms to broaden outreach
Potential Use Cases and Benefits
Encourage prior customers to provide feedback through incentives
Increase the volume of positive reviews to attract new customers
Strengthen customer relationships by recognizing their contributions
Gather valuable insights on products/services for continuous improvement
Create a buzz around new launches through enhanced online presence
Utilizing the Review Day Voucher feature addresses the common challenge of engagement in today's competitive market. By rewarding customers for their reviews, you not only enhance their experience but also increase your business's credibility. This approach turns feedback into an opportunity, helping to grow your reputation and encourage repeat purchases.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do I get a priority review voucher?
Eligible candidates are granted two vouchers and receive priority review for each voucher: the drug winning a voucher for a neglected or rare disease, and the drug using a voucher for another indication. By moving a drug to faster review, there is the potential to slow other drugs.
What is an FDA priority review voucher?
Priority review. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for drugs that might otherwise not be profitable to develop because of a smaller pool of patients needing treatment.
How much is a priority review voucher worth?
The highest price paid for a priority review is $350 million in August 2015, when United Therapeutics sold its voucher to Abbie. And in May 2015 Retrofit sold a PRV, originally transferred from Asclepius Pharma, to Sanofi for $245 million.
What does FDA priority review mean?
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. When seeking approval for a drug, manufacturers can apply to the FDA for priority review.
How long does FDA priority review take?
Priority Review means that the time it takes FDA to review a new drug application is reduced. The goal for completing a Priority Review is six months.
What is the FDA fast track for drug approval?
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs which treat a serious or life-threatening condition and fill an unmet medical need. Fast Track designation must be requested by the drug company.
How long does FDA fast track approval take?
Fast Track Process The drug developer must request the Fast Track designation. The request can be made at any time during the development process and the FDA will review and make a decision within sixty days.
What is the biggest difference between a New Drug Application NDA seeking standard approval versus accelerated approval?
The difference is that drugs granted accelerated approval must promptly conduct post-marketing confirmatory trials to verify the clinical benefit (as early as underway at the time the marketing application is submitted).
#1 usability according to G2
Try the PDF solution that respects your time.






