Rework Us Contact Title For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
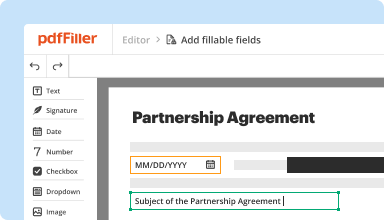
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
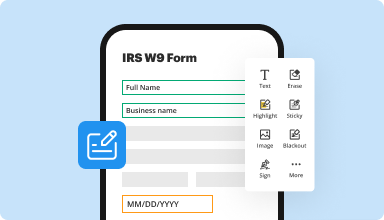
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
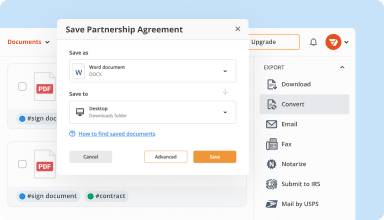
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
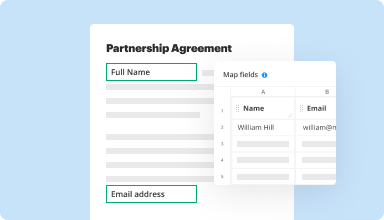
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
I have struggled trying to fill in 1099 forms for 3 days. Decided to Google for a template. Found PDFfiller and I am over the moon on how easy it is to use. Love it!
2015-01-28
Its a great tool. I use it and will continue to. The price seems steep but I bought it for a year, so I guess it's in my price range. Still wish it was cheaper. I'll admit I'm a penny puncher though. So I can be a cheap one.
2018-07-09
Its been great. I had an important application to complete and because of this software, I was successfully in completing it and looking professional at the same tim.
2019-03-14
This form filler has been incredibly beneficial in aiding me to efficiently complete a number of form related tasks....I would unequivocally recommend this software to all college student!!!
2019-09-14
very happy so far
very happy so far, awesome program for what I'm doing, fairly easy to navigate thus far, the real test will come in a few months when I'm using it a bit more to its potential.
2019-03-08
customer support
I had cancelled my subscription but it had not gone through and was charged. I explained this to the customer support team and they helped me very nicely and gave me a refund. It was efficient and very understanding!
2023-10-01
I was perplexed when my money was…
I was perplexed when my money was deducted without prior notice. I didn't know what to do at first because I thought that refund couldn't be possible but I decided to reach out to them despite the uncertainty.
I was happy with the services rendered, the customer relationship and grateful for the refund. The service is customer friendly and has quick response to issues.
2021-12-10
There was a bit of a learning curve to getting the software's capabilities down, but once I understood how to use the forms feature and whatnot, I find I really enjoy using your software more than I even like Adobe. I think your program has better templates and features than the others I have tried. Bonus points for being lower priced than Adobe while you're at it.
2021-02-01
WE HAVE a issue because date of birth format used by pdf filler is mm/dd/yyyy whereas in Australia all forms use dd/mm/yyyyy format. send me a solution tip.
2025-03-18
Rework Us Contact Title Feature
The Rework Us Contact Title feature simplifies the way you manage your team and organization. This tool allows you to customize and update contact titles across your workspace effortlessly. It enhances clarity and improves communication within your team by ensuring everyone understands each role.
Key Features
Easily update contact titles in real-time
Customize titles for clarity across teams
Streamlined organization of roles and responsibilities
Access role titles from any device with ease
Potential Use Cases and Benefits
Improve team dynamics by clarifying roles
Promote a professional image by using updated titles
Facilitate better communication within departments
Enhance onboarding processes with clear role definitions
This feature can help you solve common problems related to miscommunication and confusion over roles. By providing clear and updated contact titles, you can ensure that everyone knows their responsibilities. This clarity can lead to more efficient workflows and an overall better experience for your team.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What is FDA CFR 21?
Title 21 is the portion of the Code of Federal Regulations that governs food and drugs within the United States for the Food and Drug Administration (FDA), the Drug Enforcement Administration (DEA), and the Office of National Drug Control Policy (ONDCP).
What is meant by 21 CFR?
Title 21 of the CFR or the Code of Federal Regulations deals with governing of food and drugs in the United States for three of its governing bodies: The FDA (Food and Drug Administration), DEA (Drug Enforcement Agency) and ONDCP (Office of National Drug Control Policy).
What is the meaning of 21 CFR?
The Code of Federal Regulations (CFR) is a codification of the general and permanent rules published in the Federal Register by the Executive departments and agencies of the Federal Government. Title 21 of the CFR is reserved for rules of the Food and Drug Administration.
Why do we have 21 CFR?
By introducing the 21 CFR Part 11 rule, the FDA have essentially enabled the Life Science community and other FDA regulated industries to streamline business processes, reduce turnaround time and costs, all by establishing standard criteria for the use of electronic records and signatures.
What does it mean to be 21 CFR Part 11 compliant?
FDA 21 CFR Part 11 compliance dictates that those companies who use electronic systems for document and signature control must provide assurance that the electronic documents are authentic. FDA 21 CFR Part 11 compliance dictates that signatures whether electronic or handwritten be linked to their respective records.
Who must comply with 21 CFR Part 11?
Practically speaking, Part 11 applies to drugmakers, medical device manufacturers, biotech companies, biologics developers, CRO's, and other FDA-regulated industries, with some specific exceptions.
What is 21 CFR Part 820 and why should you care?
21 CFR Part 820 Ensures Your Device is Safe and Effective The purpose of regulatory affairs is to ensure that your company complies with applicable laws and regulations. These regulations, such as Quality System Regulation 21 CFR Part 820, are intended to ensure devices entering the marketplace are safe and effective.
What is the difference between ISO 13485 and 21 CFR 820?
ISO 13485 is a global standard that is voluntary in the US but required in some countries. ISO 13485 will become the FDA's mandatory RMS April 2019. 21 CFR 820 is applicable to manufacturers of finished medical devices sold in the United States, including imported products.
#1 usability according to G2
Try the PDF solution that respects your time.






