Split Date Accreditation For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
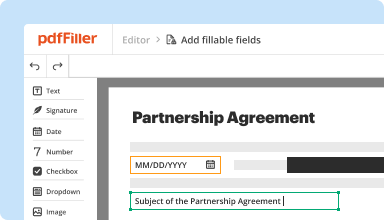
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
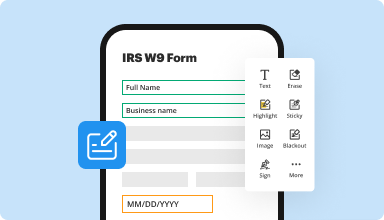
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
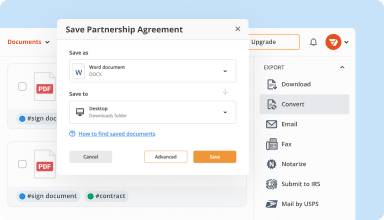
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
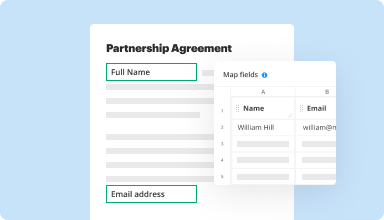
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
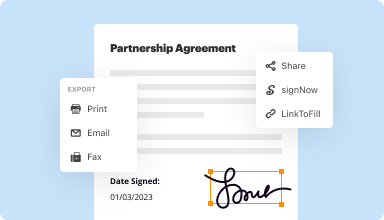
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
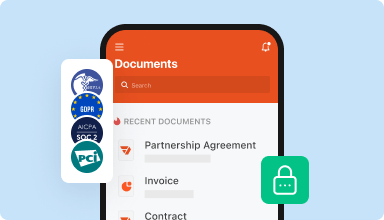
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
Excellent. Being able to type into a pdf document, email, fax, etc. has been the greatest experience! Thank you PDF filler. you are the answer to my prayers.
2017-04-11
I have been looking for a "filler" and to date have never been satisfied until I came across PDF Filler. So, far it has more than met any expectation I would have had and I look forward to using it in the future.
2018-04-18
PDF editor PDF filler
Give it a go to find out what it has in store for you
I love this product, it is readily available on the internet can work a handful of pages to correction without downloading this software.
Cant think of many cons it works best for me to do my general corrections, might not have detailed features for a professional editor, but definitely works for me.
2018-10-16
Superb customer support
Just want to give a shout out to Zel from Customer Support. Very helpful in resolving the issue I had and was very understanding. Prompt responses with a satisfied resolution.
2024-05-03
WEGTRUIHSGIDBSFJGKFSABIUJKJFKJHSDB…
WEGTRUIHSGIDBSFJGKFSABIUJKJFKJHSDB FKJGASVEJBKVSHOIGKREALJHGDVBJKWQ6T58934RHGJKJH65OP4IURY874Y3HITUGFRNT'JO4IYGFIHOWGY56HUOPWITU98TYHFN POMUYV89MU9
2022-05-11
It is useful. Only thing I would like is for it to figure out what font I had on the document BEFORE I edit it. I have contract templates that I use that I have to edit sometimes and finding the font that will match that size and actual type is very difficult. i usually end up just dealing with whichever one I find. If there is a way to do that, please let me know via email.
2021-11-02
this is the best product I could find…
this is the best product I could find for converting a pdf form into something fillable. I don't need to use this regularly so better if you offered a annual usage limit package- say 12 uses a year for $12.00- which seems fair and reasonable to you and the customer. (If you take this idea up, please do let me know)Ross Harling
2021-06-17
What do you like best?
The ability to upload documents as templates, to work on completed documents, whether changing page order or add/modify information.
What do you dislike?
I find it challenging to change fonts and text color. I'm not even sure it's possible.
What problems are you solving with the product? What benefits have you realized?
Simplification of creating clear, typed forms that elevate the professionalism of everythign we do.
2021-02-16
Clueless in North Carolina!
I was clueless as to how to fill out the tax documents for my 1099 employees and your company walked me through it and I accomplished the task confidently.
2025-03-12
Split Date Accreditation Feature
The Split Date Accreditation feature offers you a flexible solution for managing accreditation dates. It allows you to separate the start and end dates for different accreditation cycles. This feature streamlines your accreditation process and helps you maintain compliance with various standards.
Key Features
Separate accreditation start and end dates for better organization
User-friendly interface for easy management
Automated notifications for upcoming accreditation deadlines
Option to apply different accreditation rules for each date
Real-time updates on accreditation status
Potential Use Cases and Benefits
Ideal for educational institutions managing multiple programs
Perfect for businesses needing to track compliance with various standards
Useful for healthcare organizations maintaining certification timelines
Streamlines reporting for audits and evaluations
Improves resource allocation for accreditation activities
With the Split Date Accreditation feature, you can easily navigate the complexities of accreditation management. By aligning your dates with your needs, you eliminate confusion and ensure compliance. This feature saves time, reduces stress, and allows you to focus on what really matters: delivering quality and maintaining standards.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
Who needs a CIA waiver?
CIA requires that any facility examining human specimens for diagnosis, prevention, treatment of a disease or for assessment of health must register with the federal Centers for Medicare & Medicaid Services (CMS) and obtain CIA certification.
Do I need a CIA waiver?
A. Yes, the testing you perform qualifies as waived laboratory testing, and you need a CIA Certificate of Waiver. This testing requires a CIA certificate regardless of how many tests you perform and even if you do not charge the patient or bill Medicare or other insurances.
Is a CIA waiver required?
Generally yes, as those tests likely qualify as waived laboratory testing, you need a CIA Certificate of Waiver and you must follow the manufacturer's instructions.
Who needs a CIA number?
According to the CIA regulations, any laboratory that conducts even a single test on “materials derived from the human body for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or the impairment of, or assessment of, the health of human beings” is required to obtain a ...
How do I get a CIA waiver certificate?
Obtaining CIA Waiver. Apply for a CIA Certificate using Form CMS-116, available through the Centers for Medicare and Medicaid Services. ...
Review your qualifications for a waiver. ...
Apply for a certificate of waiver. ...
Develop a quality assurance plan.
What does CIA Waiver mean?
As defined by CIA, waived tests are simple tests with a low risk for an incorrect result. They include: Certain tests listed in the CIA regulations. ... Tests that the manufacturer applies to the FDA for waived status by providing scientific data that verifies that the CIA waiver criteria have been met.
How do I get a CIA waiver?
Obtaining CIA Waiver. Apply for a CIA Certificate using Form CMS-116, available through the Centers for Medicare and Medicaid Services. ...
Review your qualifications for a waiver. ...
Apply for a certificate of waiver. ...
Develop a quality assurance plan.
What tests are considered CIA waived?
Waived tests include test systems cleared by the FDA for home use and those tests approved for waiver under the CIA criteria. Although CIA requires that waived tests must be simple and have a low risk for erroneous results, this does not mean that waived tests are completely error-proof.
How much is a CIA waiver?
The fee for a Certificate of waiver is $150. For additional information or assistance in filling out the CIA waiver application, please call CMS Toll-Free: 877-267-2323; Local: 410-786-3000; TTY Toll-Free: 866-226-1819; TTY Local: 410-786-0727. Or visit the CIA Website.
How long does it take to get a CIA waiver?
Depending on your state health department, it can take between 4-12 weeks to receive your CIA certificate. The CIA certificate of waiver is necessary for state and federal compliance, and provides the benefit of facilitating provider reimburse- meet for the use of CLIA-waived tests.
#1 usability according to G2
Try the PDF solution that respects your time.






