Supply Formula Record For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
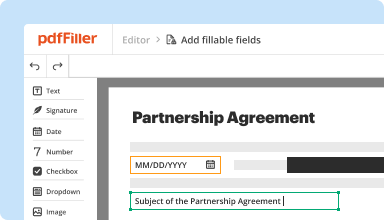
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
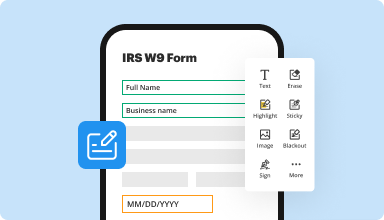
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
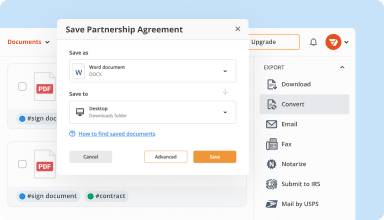
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
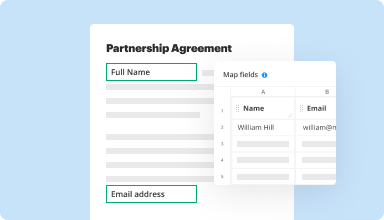
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
I just started to use PDFfiller and like the ease of completing pdfs, and logical flow of the app! Also love the send fax feature although while it seemed to work well from the desktop app, it seemed to hang when faxing from my galaxy Note 4 (just kept spinning and I had to force stop the app)-- will have to try faxing more to see if it was a device or app issue.
2016-07-28
The forms are easy to find with the search tool, and very easy to use. There are even pop-ups to tell you what type of information to enter in the different fill-in fields. I'm sure I will be using more forms in the future here.
2016-11-28
Very Satisfied and excellent customer service. After a charge dispute, the error was fixed immediately. Then had a print problem and that problem was corrected before I could explain to CS. Great service!!!!
2017-05-24
What do you like best?
No need to print at all! Completely paperless
What do you dislike?
Everything is perfect no negative comments
Recommendations to others considering the product:
Great on completing and signing PDF’s
What problems are you solving with the product? What benefits have you realized?
Complete all forms on a go !! Saves time and money on printing cartridge
No need to print at all! Completely paperless
What do you dislike?
Everything is perfect no negative comments
Recommendations to others considering the product:
Great on completing and signing PDF’s
What problems are you solving with the product? What benefits have you realized?
Complete all forms on a go !! Saves time and money on printing cartridge
2019-10-07
pdf Filler is a very useful option when…
pdf Filler is a very useful option when you need other people's signatures, or want to modify documents with pre-filled information. Have used on/off for 15 years
2024-06-18
the company is very nice and it looks…
the company is very nice and it looks like a place that you will put your trust in and i can see it can help and i really thank the company and i love everyone that is in this site
2023-09-06
So helpful. I can fill out the form instead of scanning, printing, filling out, and then scanning again so that I can send it back. I did try other programs... this one is by far the very best.
2023-03-30
Pdf Filler and the support…
Pdf Filler and the support representative Dee went above and beyond to make sure we tried all options to make sure I was able to manipulate the document exactly the way I wanted. Thanks again!
2022-02-21
What do you like best?
It is easy to use you can upload and send document for signatures.
It easy to edit and add information or make changes to any pdf documents.
What do you dislike?
No complaints very good software if any problems the PdfFiller team will send an email to fix it.
Recommendations to others considering the product:
Get it as soon as possible the best pdf editor ever.
What problems are you solving with the product? What benefits have you realized?
Being able to edit pdfs and sign electronically
2021-07-21
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do you make a master formula record?
Master formula record (MFR) is a master document for any pharmaceutical product. A MFR should have following parts: Product Details: First on the first page following details about the product are added. Name, logo and address of the manufacturing company. Dosage form name. Brand name. Generic name. Product code.
What is a master formula?
MASTER FORMULA — A document or set of documents specifying the raw materials with their quantities and the packaging materials, together with a detailed description of the procedures and precautions required to produce a specified quantity of a finished product.
What is master formula in pharmacy?
MASTER FORMULA RECORD Master Formula Record (MFR) is a master document for any pharmaceutical product. MFR contains all information about the manufacturing process for the product. MFR is used as reference standard for preparing batch manufacturing record (BMR) by manufacturing units.
What is a master batch document?
Definition of Master Batch Record Master Batch Record means the document that defines the manufacturing methods, materials, and other procedures, directions and controls associated with the manufacture and testing of the Product, which may be amended in writing from time to time by mutual agreement of the parties.
What is batch formula record?
Batch manufacturing record is a written document of the batch, prepared during pharmaceutical manufacturing process. It contains actual data and step-by-step process for manufacturing each batch. Batch manufacturing record is like a proof that batches were properly made and checked by quality control personnel.
What is a master manufacturing record?
Master Manufacturing Record means, for each Project, the template document proposed by Paragon and approved by Client that defines the final and complete manufacturing methods, test methods, materials, and other procedures, directions and controls associated with the manufacture and testing of Product.
What is master batch record?
Master Batch Records (Mrs) are general manufacturing instructions. Batch Records are derived from them and refer to a specific order. Master batch records and batch records are therefore the basis for a precise and detailed description of pharmaceutical manufacturing processes.
What are batch manufacturing records?
Batch manufacturing record is a written document of the batch, prepared during pharmaceutical manufacturing process. It contains actual data and step-by-step process for manufacturing each batch. Batch manufacturing record is like a proof that batches were properly made and checked by quality control personnel.
#1 usability according to G2
Try the PDF solution that respects your time.






