Word Application For Medical Device Manufacturers Online For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
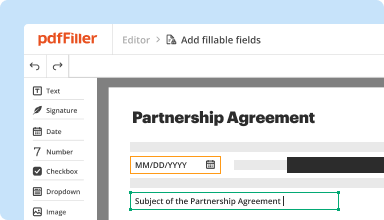
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
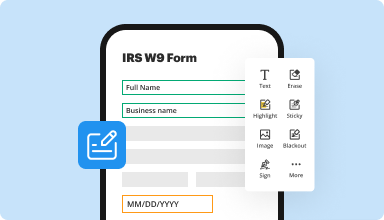
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
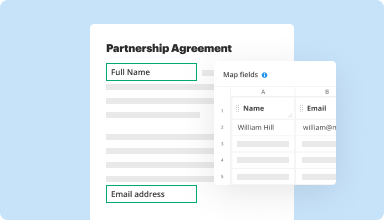
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
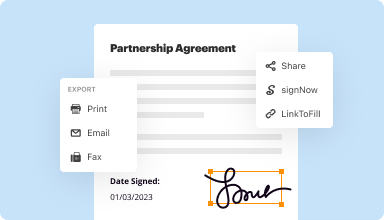
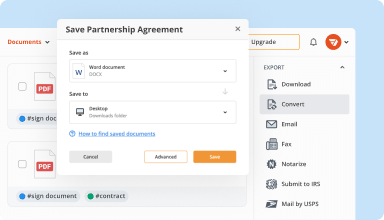
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
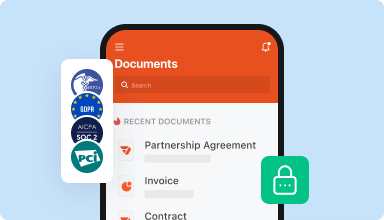
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
I believe the staff is dedicated to helping the customers and are very professional. Thought I might have to talk to them in person, but they answer and respond quickly through chat and do everything they can to help you right away. When I just had to get my material out right away and short on funds allowed me to try out the program just a few days after I explained my difficulty. Then after few days able to of course, pay for this program that has helped me so much. The forms are easier to read by using PDFfiller, and the video of how to work the software is helpful as well. The people are just awesome!! Very helpful and respond immediately. I would recommend this to friends and family as well.
2015-03-12
easy to use , quick , efficient saves me time . switched from ADOBE which WAS a good product but no more. crashed so often that I could barely complete one report in a day. I did this one in less than an hour. Thank you
2018-10-22
His is getting much easier now that I've done it a couple times and it is very convenient to have an Online service so I can use any of my Electronic devices.
2019-01-04
Over all really helpful, especially with me filling out awards and scholarships for grade 12. Only issue I had was when typing the text box wouldn't fit as nicely as I would have liked it to, so lines ran through the text and made it a bit harder to read.
2019-05-01
I love this app
I love this app, it makes sure to compensate for all the things which you would need to do for schoolwork. Personally though, I think it would make more sense to market it with ads rather than subscription. This is really useful for students who are homeschooled or aren't able to go to school and students usually don't have much in their pockets to pay for these kinds of things.
2020-03-26
A bit difficult to navigate…
A bit difficult to navigate through/enter updates unless you use this on a regular basis. Once the memory kicks in, it's a GREAT tool for making easy changes/adding necessary information.
2020-01-21
Great onboarding, and lifesaving functionality
Lifesaver, amazing and frictionless free sign-up journey. Edited and saved a really important PDF in minutes
2024-08-05
I have used pdffiller for 5 years, they are an invaluable service. I have a tax and accounting firm, could not run my business without them. The service department is very helpful, and get back to you with a day. *************************** - *********, **
2023-03-21
Ryan on the Support Team was extremely helpful and patient. He walked me through all of the steps to complete the form to my satisfaction. Thank you Ryan for teaching me!
2020-05-21
Word Application for Medical Device Manufacturers
The Word Application for Medical Device Manufacturers simplifies documentation processes for your business. You can streamline your workflow and ensure compliance with regulations while producing quality documents.
Key Features
Easy-to-use interface for efficient document creation
Built-in templates designed for medical device standards
Collaboration tools for team input and reviews
Automated formatting to meet regulatory requirements
Export options for various file formats
Potential Use Cases and Benefits
Create manuals and product documentation quickly
Maintain consistent branding across all documents
Facilitate team collaboration on projects and proposals
Ensure traceability and compliance for audits
Reduce time spent on document preparation and revisions
With the Word Application, you can tackle the common challenges of documentation in the medical device industry. It helps you save time, improve accuracy, and enhance teamwork. By using this tool, you can focus more on innovation while ensuring that your documentation meets all necessary standards.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
Does GMP apply to medical devices?
FDA has determined that certain types of medical devices are exempt from GMP requirements. Medical devices manufactured under an investigational device exemption (IDE) are not exempt from design control requirements under 21 CFR 820.30 of the QS regulation.
What are GMP requirements?
Good manufacturing practices (GMP) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices.
What are GMP standards?
Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product.
How do I become GMP certified?
Acquiring GMP Certification The application for GMP certification has to be made by an authorized person within the company seeking the certification. This is usually one with responsibility such as a Production Manager, a Quality Assurance Manager, a Quality Control Manager or the Managing Director.
What are the 5 main components of good manufacturing practice?
To simplify this, GMP helps to ensure the consistent quality and safety of products by focusing attention on five key elements, which are often referred to as the 5 P's of GMP people, premises, processes, products and procedures (or paperwork). And if all five are done well, there is a sixth P profit!
#1 usability according to G2
Try the PDF solution that respects your time.






