Generator Software with pdfFiller
Discover how to easily create a Clinical Evaluation Medical Device Report Template using pdfFiller’s generator software. This powerful tool allows you to efficiently generate documents, customize formats, and share files seamlessly.
What is a Clinical Evaluation Medical Device Report Template?
A Clinical Evaluation Medical Device Report Template is a structured document utilized to capture essential data and findings regarding the clinical evaluation of medical devices. It serves as a formal report that consolidates information on safety, efficacy, and performance based on clinical investigations or literature reviews. These templates are crucial for meeting regulatory requirements, ensuring compliance, and guiding decision-making within organizations and clinical settings.
Why you might need to create a Clinical Evaluation Medical Device Report Template
Creating a Clinical Evaluation Medical Device Report Template is vital for various reasons:
-
1.Ensures compliance with regulatory standards set by bodies such as the FDA and EMA.
-
2.Facilitates documentation of clinical evidence and study outcomes.
-
3.Enhances communication among stakeholders involved in the device's lifecycle.
-
4.Improves the organization and clarity of complex data concerning medical devices.
Key tools in pdfFiller that let you generate a Clinical Evaluation Medical Device Report Template
pdfFiller offers multiple features designed to simplify the creation of Clinical Evaluation Medical Device Report Templates:
-
1.Customizable templates: Start from scratch or modify pre-existing templates according to your needs.
-
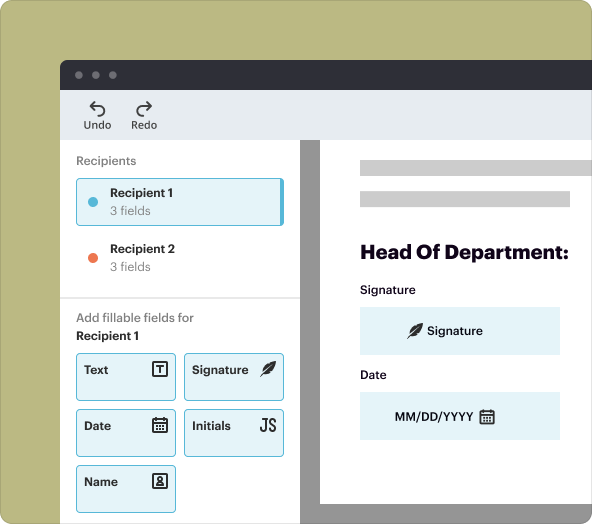
2.Collaborative editing: Invite team members to review and input data in real-time.
-
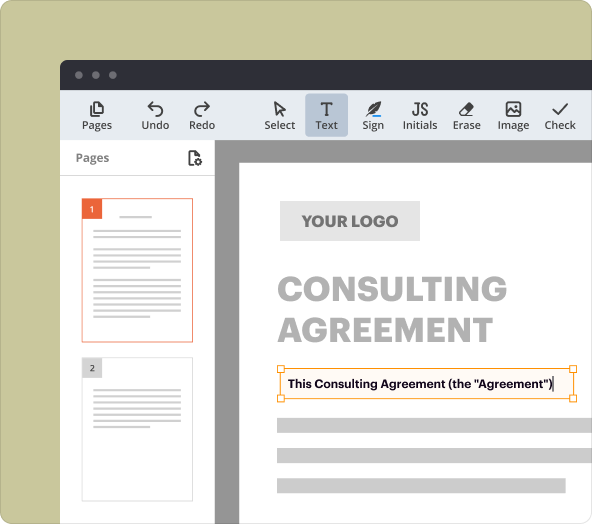
3.PDF editing and annotation: Easily edit text, add images, and include comments or highlights.
-
4.Seamless eSignature capability: Collect digital signatures directly within the document.
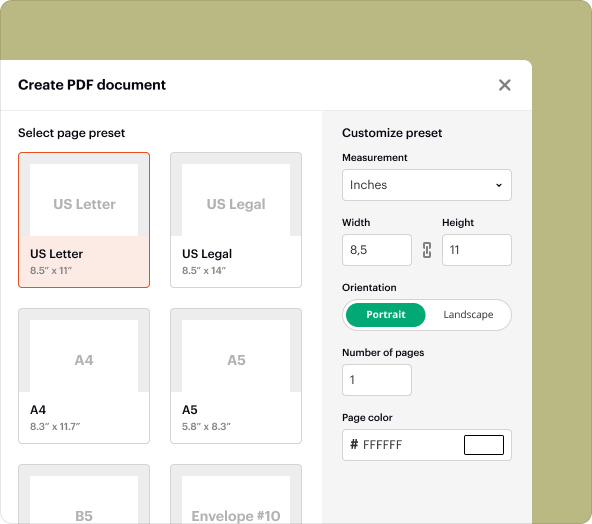
Step-by-step guide to creating blank PDFs for Clinical Evaluation Medical Device Report Template
Creating a blank PDF using pdfFiller is straightforward:
-
1.Log in to your pdfFiller account.
-
2.Select the "Create Document" option from the dashboard.
-
3.Choose "Blank Document" to initiate a new PDF.
-
4.Add fields, text boxes, and other necessary components per your template requirements.
-
5.Save your newly created template for future use.
Clinical Evaluation Medical Device Report Template from scratch vs uploading existing files to modify
When generating a Clinical Evaluation Medical Device Report Template, you have the option to start fresh or modify existing documents:
-
1.Starting from scratch allows for complete customization, ensuring the report meets specific needs.
-
2.Uploading existing files can save time, especially if you have relevant documents that only need minor adjustments.
Organizing content and formatting text as you create a Clinical Evaluation Medical Device Report Template
Efficiently structuring and formatting your report is essential for clarity:
-
1.Utilize headings and subheadings for different sections of the report, such as Introduction, Methods, Results, and Conclusion.
-
2.Ensure a consistent font type and size throughout the document.
-
3.Use bullet points and numbered lists to convey information clearly and concisely.
-
4.Incorporate tables or charts for presenting data visually, making complex information easier to digest.
Saving, exporting, and sharing once you create a Clinical Evaluation Medical Device Report Template
Once you have finalized your report, you can save, export, and share it with ease:
-
1.Click “Save” to secure your document in the cloud, allowing access anytime, anywhere.
-
2.Export the document to various formats, including PDF, Word, and Excel, based on your needs.
-
3.Utilize the sharing features to email the report directly from pdfFiller or generate a shareable link for collaborators.
Typical use-cases and sectors that often utilize a Clinical Evaluation Medical Device Report Template
Various industries benefit from utilizing Clinical Evaluation Medical Device Report Templates, including:
-
1.Healthcare organizations conducting clinical trials or evaluations.
-
2.Medical device manufacturers needing documentation for regulatory submissions.
-
3.Consultancy firms offering expertise in regulatory affairs and clinical evaluations.
-
4.Research institutions and academic entities engaged in medical device studies.
Conclusion
Utilizing pdfFiller's generator software to create a Clinical Evaluation Medical Device Report Template streamlines your documentation process, enhances collaboration, and ensures compliance with regulatory standards. By leveraging its powerful features, you can effectively produce, edit, and share professional reports from anywhere, optimizing efficiency for individuals and teams alike.
How to create a PDF with pdfFiller
Who needs this?
Document creation is just the beginning
Manage documents in one place
Sign and request signatures
Maintain security and compliance
pdfFiller scores top ratings on review platforms