How to Informed in Research Consent Template with pdfFiller
Creating an Informed in Research Consent Template is crucial for ensuring compliance and ethics in research practices. With pdfFiller, you can effortlessly generate, modify, and manage consent templates tailored to your specific needs. This guide provides detailed steps for using pdfFiller's powerful features to create your informed consent documents.
What is an Informed in Research Consent Template?
An Informed in Research Consent Template is a crucial document that outlines the details of a research study, allowing participants to understand what is expected of them and how their data will be used. This document ensures transparency and builds trust between researchers and participants. Such templates typically include information regarding the study's purpose, procedures, risks, benefits, and the participants' rights.
Why you might need to create an Informed in Research Consent Template?
Organizations often require Informed in Research Consent Templates to comply with ethical standards and legal regulations. These templates ensure that all research complies with institutional review boards (IRBs) and protects participants' rights. By having a well-crafted consent template, researchers can avoid potential legal issues, enhance participant recruitment, and contribute to responsible research practices.
Key tools in pdfFiller that let you create an Informed in Research Consent Template
pdfFiller offers numerous functionalities to streamline the process of creating Informed in Research Consent Templates. Key features include:
-
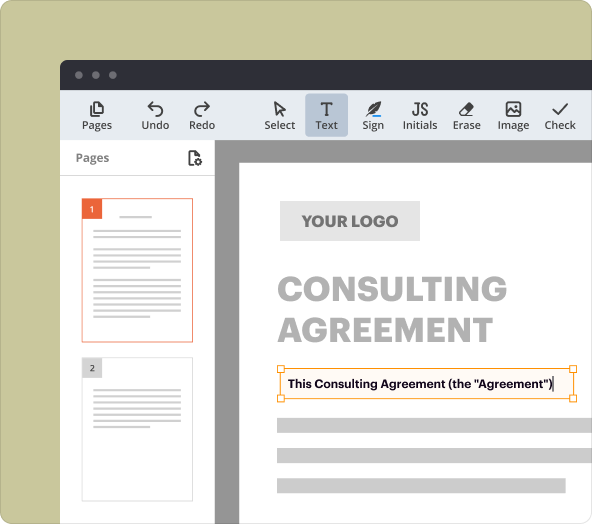
1.Document Editor: Easily edit and customize your templates to fit specific research requirements.
-
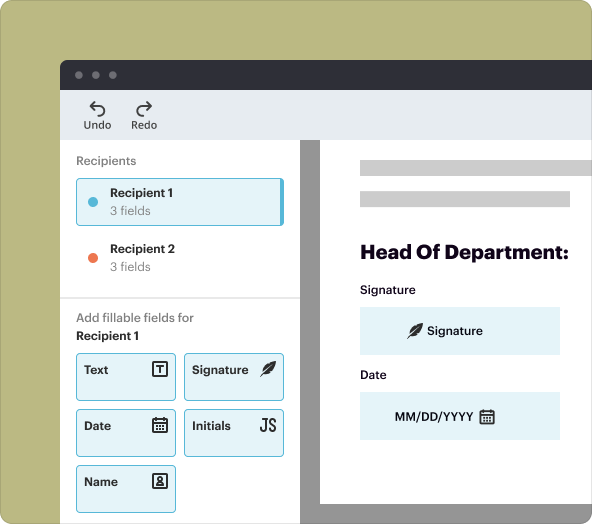
2.eSignature: Collect electronic signatures securely from participants to validate consent.
-
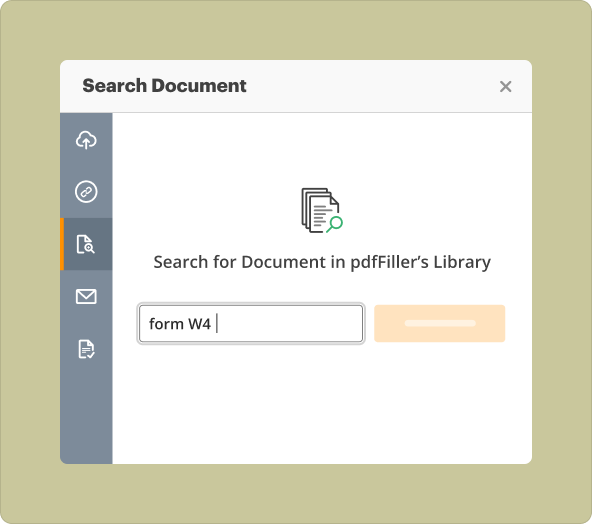
3.Templates Library: Access a wide range of pre-existing templates to accelerate the creation process.
-
4.Collaboration Tools: Allow team members to collaborate on document creation, ensuring comprehensive and accurate information.
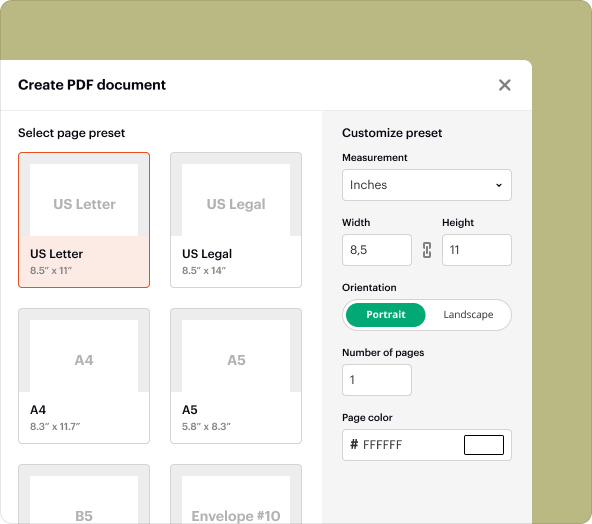
Step-by-step guide to create blank PDFs
Creating a blank PDF consent template in pdfFiller is straightforward. Follow these steps:
-
1.Log into your pdfFiller account.
-
2.Navigate to the Document Management section.
-
3.Select "Create New Document" and choose "Blank PDF."
-
4.Use the editing tools to add text fields, checkboxes, and signature areas.
-
5.Customize the template according to your research needs.
Creating an Informed in Research Consent Template from scratch vs uploading existing files to modify
When using pdfFiller, you have the option to start from scratch or upload existing documents. Each method has its advantages:
-
1.From Scratch: Offers the flexibility to design your template from the ground up, ensuring it fully meets your requirements.
-
2.Upload Existing Files: Ideal for modifying or updating current templates, saving time and effort while retaining essential information.
Organizing content and formatting text as you create an Informed in Research Consent Template
Structure and formatting are critical in consent templates for clarity and professionalism. Here’s how to effectively organize content in pdfFiller:
-
1.Utilize headings and subheadings for easy navigation.
-
2.Employ bullet points and numbered lists for key information.
-
3.Adjust font size and style to ensure readability.
-
4.Incorporate tables if needed to present data clearly.
Saving, exporting, and sharing once you create an Informed in Research Consent Template
Once your consent template is complete, pdfFiller offers several options for saving and sharing:
-
1.Save in the Cloud: Store your template in your pdfFiller account for easy access anytime.
-
2.Export Options: Download your consent template in various formats, including PDF, DOCX, or JPEG.
-
3.Share Functionality: Send the document directly to participants or team members via email or a shareable link.
Typical use-cases and sectors that often require Informed in Research Consent Template
Various sectors utilize Informed in Research Consent Templates, including:
-
1.Healthcare: Medical studies that involve patient data and treatments.
-
2.Academic Research: Universities conducting surveys or experiments involving human subjects.
-
3.Clinical Trials: Pharmaceutical companies testing new drugs or devices.
-
4.Social Science: Research involving participant interviews or observations.
Conclusion
Creating an Informed in Research Consent Template using pdfFiller is a streamlined process that enables researchers to maintain compliance and uphold ethical standards. With a variety of powerful tools, intuitive editing capabilities, and flexible options for collaboration, pdfFiller is the ideal solution for individuals and teams seeking a comprehensive document management platform. Start creating your consent templates today for effective and ethical research practices.
How to create a PDF with pdfFiller
Who needs this?
Document creation is just the beginning
Manage documents in one place
Sign and request signatures
Maintain security and compliance
pdfFiller scores top ratings on review platforms




I can take forms from online and my computer and can easily and neatly fill them in. I also love the form creation feature.
What do you dislike?
Learning to create forms is much harder than expected.
Recommendations to others considering the product:
Great service. Uploading and using forms is intuitive and easy. Allow time for learning if creating own forms.
What problems are you solving with the product? What benefits have you realized?
I mainly use it to complete and store forms. I plan to use it to create forms in the future.