Irb Informed Consent Template Builder Solution with pdfFiller
Creating an Irb Informed Consent Template is simple with pdfFiller’s Builder Solution, designed to help individuals and teams efficiently design and manage PDF documents. This comprehensive tool allows users to generate and modify consent forms, ensuring compliance with Institutional Review Board (IRB) requirements, while also streamlining document workflows.
What is an Irb Informed Consent Template?
An Irb Informed Consent Template is a standardized document that outlines how researchers should inform participants about the details of a study. It covers aspects such as the purpose of the research, potential risks, benefits, and the rights of participants. The IRB reviews these templates to protect the welfare of research subjects and ensure ethical standards. Using a template ensures that essential information is presented clearly and consistently.
Why you might need to create an Irb Informed Consent Template?
Creating an Irb Informed Consent Template is crucial for several reasons. First, it standardizes the consent process, making it easier for participants to understand what they are agreeing to. Second, it helps institutions meet regulatory compliance standards, which is essential for ethical research. Finally, it saves time by providing a foundation that researchers can modify according to their specific study needs while ensuring all necessary elements are included.
Key tools in pdfFiller that let you create an Irb Informed Consent Template
pdfFiller offers a variety of features tailored to streamline the document creation process. Important tools include:
-
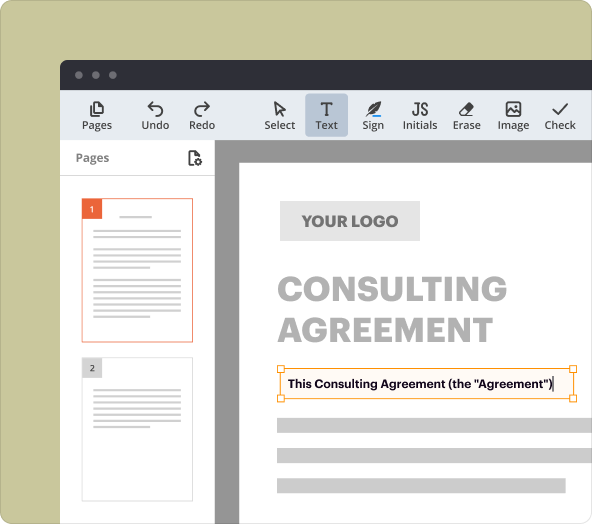
1.Document Builder: Create forms from scratch or edit existing PDFs.
-
2.Text Editing Tools: Customize text and format in your template.
-
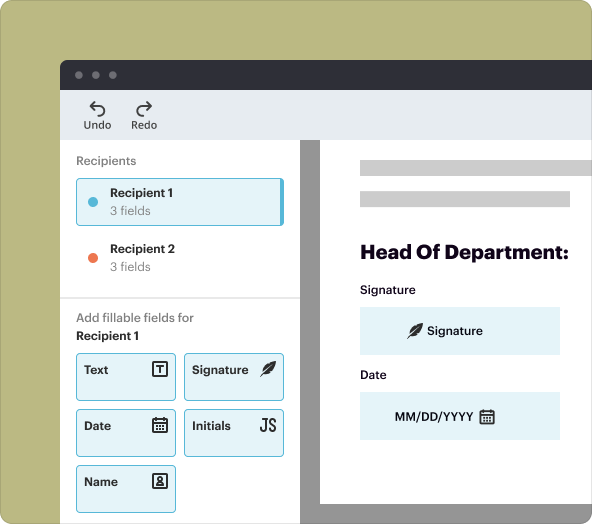
3.eSignature Functionality: Ensure that consent is properly documented with secure signatures.
-
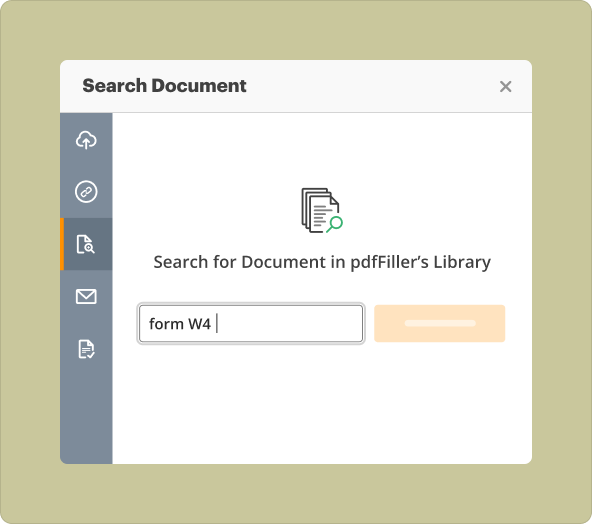
4.Cloud Storage: Access your templates from anywhere, ensuring constant availability.
-
5.Collaboration Features: Share with team members for review and input.
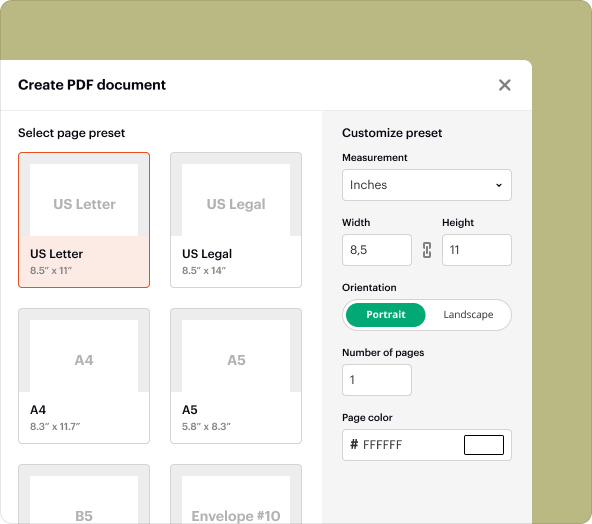
Step-by-step guide to create an Irb Informed Consent Template from blank PDFs
To create an Irb Informed Consent Template using pdfFiller, follow these steps:
-
1.Log into your pdfFiller account.
-
2.Navigate to the Document Builder option.
-
3.Select "Create New Document" to start with a blank template.
-
4.Add headings and sections based on the standard IRB template structure.
-
5.Utilize text editing tools to input content related to your study.
-
6.Save your document for further editing.
Creating an Irb Informed Consent Template from scratch vs uploading existing files to modify
When deciding between creating a document from scratch or modifying an existing one, consider the pros and cons:
-
1.Creating from Scratch: Offers full customization, but may require more time to ensure all elements are covered.
-
2.Modifying Existing Files: Saves time and ensures compliance with established standards, but may limit unique branding or specific content needs.
Organizing content and formatting text as you create an Irb Informed Consent Template
Proper formatting is essential for clarity and professionalism. Use bullet points for lists, headings for sections, and clear language to enhance comprehension. pdfFiller’s formatting tools allow you to adjust text size, font, and colors, making it easy to present information in an orderly manner.
Saving, exporting, and sharing once you create an Irb Informed Consent Template
Once your Irb Informed Consent Template is finalized, pdfFiller makes saving and sharing straightforward:
-
1.Click on the "Save" button to store the document in your account.
-
2.Choose to export the template as a PDF for printing or distribution.
-
3.Use the "Share" feature to send the document directly to participants or collaborators via email.
Typical use-cases and sectors that often use Irb Informed Consent Templates
Various sectors utilize Irb Informed Consent Templates including:
-
1.Medical and clinical research: To ensure participants are informed of their rights and risks.
-
2.Academic institutions: For studies involving student participants.
-
3.Healthcare professionals: When conducting research on patient treatments.
-
4.Social sciences: For studies involving human subjects in surveys or experiments.
Conclusion
In summary, the Irb Informed Consent Template Builder Solution from pdfFiller simplifies the process of creating and managing critical consent documents. By leveraging pdfFiller's robust tools, users can ensure their templates are compliant with IRB guidelines while being customizable to meet specific research needs. The ease of access, collaborative features, and efficient exporting capabilities make pdfFiller an essential resource for researchers and institutions alike.
How to create a PDF with pdfFiller
Who needs this?
Document creation is just the beginning
Manage documents in one place
Sign and request signatures
Maintain security and compliance
pdfFiller scores top ratings on review platforms