Build PDF forms with pdfFiller’s Adverse Event Reporting Form Creator
What is an Adverse Event Reporting Form Creator?
An Adverse Event Reporting Form Creator is a digital tool that facilitates the design and management of custom PDF forms specifically for gathering information on adverse events. These forms are vital in healthcare and pharmaceutical settings, allowing organizations to collect critical data on patient safety and treatment outcomes.
-
Helps create customizable forms for reporting adverse events.
-
Streamlines the data collection process in clinical environments.
-
Supports compliance with regulatory requirements.
How does an Adverse Event Reporting Form Creator change document preparation?
The creation of adverse event reporting forms has traditionally involved cumbersome paper-based processes. By utilizing pdfFiller’s Adverse Event Reporting Form Creator, teams can significantly reduce preparation time and improve accuracy. This platform enables users to quickly edit, store, and share forms securely online.
-
Eliminates the need for physical paperwork.
-
Increases collaboration among stakeholders, such as healthcare providers and regulatory bodies.
-
Streamlines the submission process, reducing the time from reporting to action.
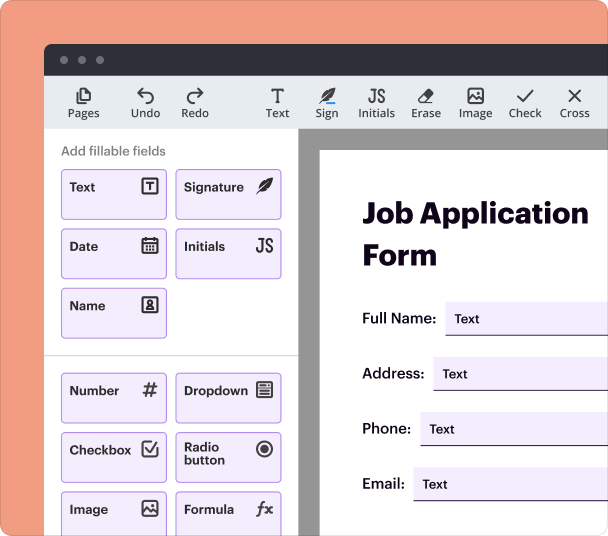
Steps to add interactive fields when creating forms
Adding interactive fields is crucial for collecting specific information efficiently. With pdfFiller, users can seamlessly add checkboxes, drop-down menus, text fields, and signature blocks to their PDF forms. This flexibility ensures that every form meets the unique requirements of the reporting process.
-
Open your PDF document in pdfFiller.
-
Select 'Add Fields' from the options menu.
-
Drag and drop the desired field types onto the document.
-
Customize field properties, including sizes and validation rules.
-
Save the form for distribution.
Setting validation and data rules as you create forms
Applying data validation rules while creating your forms ensures that submitted data is accurate and complete. The pdfFiller platform allows users to set specific criteria for each field, such as mandatory fields or predetermined format types, which enhances the quality of data collected.
-
Define which fields are mandatory for submission.
-
Establish rules for data formats (e.g., date, email).
-
Use conditional logic to guide users through the form.
Going from a blank page to finished form with an Adverse Event Reporting Form Creator
Transforming a blank page into a functioning adverse event reporting form involves a few straightforward steps within the pdfFiller platform. Users can start from scratch or utilize a template, making the process adaptable and efficient.
-
Choose to create a new form or edit an existing template.
-
Add necessary fields for gathering adverse event details.
-
Apply validation rules to ensure data accuracy.
-
Preview the form to check for usability.
-
Save and publish the completed form.
Organizing and revising templates when using an Adverse Event Reporting Form Creator
Maintaining organized templates is crucial for quick access and updates. pdfFiller allows users to categorize and edit their forms easily, ensuring that modifications keep pace with evolving regulatory demands.
-
Create folders to organize forms based on categories (e.g., department, project).
-
Edit existing templates as needed using the intuitive interface.
-
Version control features help in tracking changes over time.
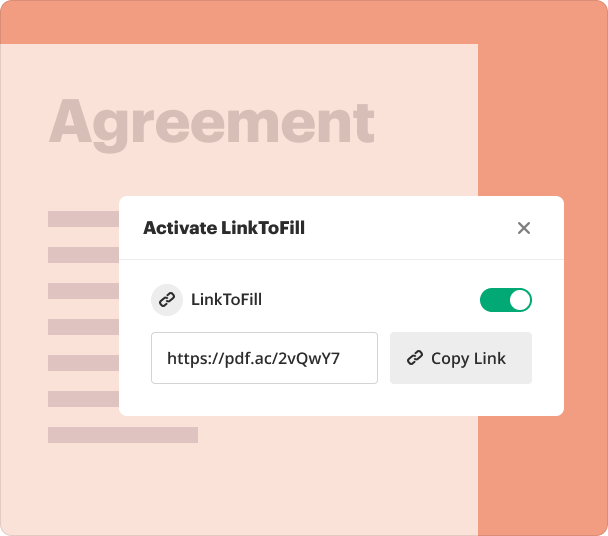
Sharing results and monitoring responses after creating forms
Once the adverse event reporting form is complete, sharing it with stakeholders is seamless through pdfFiller’s sharing capabilities. Users can track submission status and responses in real-time, enhancing accountability and follow-up efforts.
-
Send forms via email or link to collaborators.
-
Monitor submissions with built-in tracking features.
-
Receive notifications of changes or updates to forms.
Exporting collected data once using the Adverse Event Reporting Form Creator
Exporting the data collected from adverse event forms is a critical step for analysis and reporting. pdfFiller allows users to transform submitted data into various formats, making it easier to integrate into existing databases or reporting systems.
-
Select the data export option from the pdfFiller dashboard.
-
Choose the format for export (e.g., CSV, Excel).
-
Download the file for use in further analysis.
Where and why businesses use an Adverse Event Reporting Form Creator
Businesses in healthcare, pharmaceuticals, and clinical research heavily rely on adverse event reporting forms for compliance and safety monitoring. pdfFiller provides a robust solution that caters to these sectors by simplifying reporting and ensuring data integrity.
-
Used in clinical trials to document adverse reactions.
-
Facilitates regulatory compliance for healthcare providers.
-
Ensures patient safety and quality assurance in treatment protocols.
Conclusion
The Adverse Event Reporting Form Creator within pdfFiller not only simplifies the form creation process but also enhances data collection, compliance, and analysis capabilities. By utilizing this powerful tool, healthcare and pharmaceutical teams can focus more on patient outcomes and less on administrative hurdles.
-
Empower teams to build effective and compliant reporting forms.
-
Facilitate seamless data management and sharing.
-
Support organizational efficiency through digital workflows.
How to create a PDF form
Who needs this?
The all-in-one PDF form creator, editor, and eSignature solution
Handle all your docs in one place
Keep data secure
Share and collaborate
pdfFiller scores top ratings on review platforms