Build PDF forms with pdfFiller’s Clinical Trial Consent Form Creator
What is a Clinical Trial Consent Form Creator?
A Clinical Trial Consent Form Creator is a tool designed to assist researchers and healthcare professionals in generating legally required consent forms for clinical trials. These forms are essential for obtaining participant permission and ensuring that they understand the risks and benefits associated with the study.
-
Streamlines the creation of complex forms.
-
Ensures compliance with legal requirements.
-
Facilitates quick revisions and updates.
How does a Clinical Trial Consent Form Creator change document preparation?
Implementing a Clinical Trial Consent Form Creator can significantly enhance document preparation processes. This tool allows users to create forms that are customizable and interactive, streamlining the paperwork involved in clinical research.
-
Automation of repetitive tasks in form creation.
-
Reduction of errors compared to manual entries.
-
Facilitated compliance with regulatory standards.
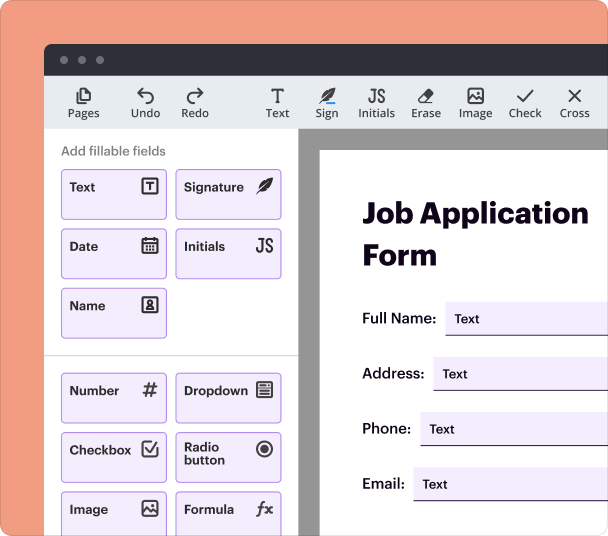
Steps to add fields when you create a consent form
Adding fields to your consent form is straightforward with the pdfFiller platform. Users can easily click and drag to place different elements where needed. Here’s how you can do it:
-
Open the form editor on pdfFiller.
-
Select the 'Add Fields' option from the toolbar.
-
Choose from text fields, checkboxes, radio buttons, etc.
-
Position fields precisely by dragging them.
-
Customize field properties like size and validation rules.
Setting validation and data rules as you create a consent form
Establishing validation rules is crucial to ensure the correctness of submitted data. You can specify the requirements for each field, making sure that all necessary information is collected.
-
Choose a field and access its properties.
-
Set mandatory field requirements.
-
Add conditions for data types, such as email addresses and dates.
-
Implement error messages for incorrect entries.
Going from blank page to finished form while you create a consent form
The pdfFiller platform allows users to swiftly go from a blank document to a fully structured consent form. The intuitive interface minimizes the learning curve and accelerates the creation process.
-
Start with a blank form or a template from the library.
-
Utilize drag-and-drop functionality to add elements.
-
Adjust properties and settings for each form field.
-
Preview the form to ensure correctness.
-
Save and publish the final version.
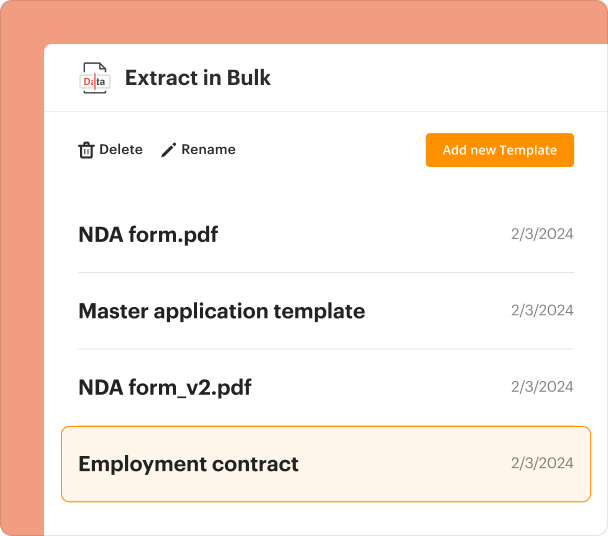
Organizing and revising templates when you create a consent form
Keeping templates organized is key for efficient workflow, especially in clinical settings where documentation often requires updates. pdfFiller allows users to efficiently manage their template library.
-
Categorize templates based on project or trial type.
-
Use tags for easy searching.
-
Version control helps track changes over time.
-
Collaborate with team members for feedback before finalizing.
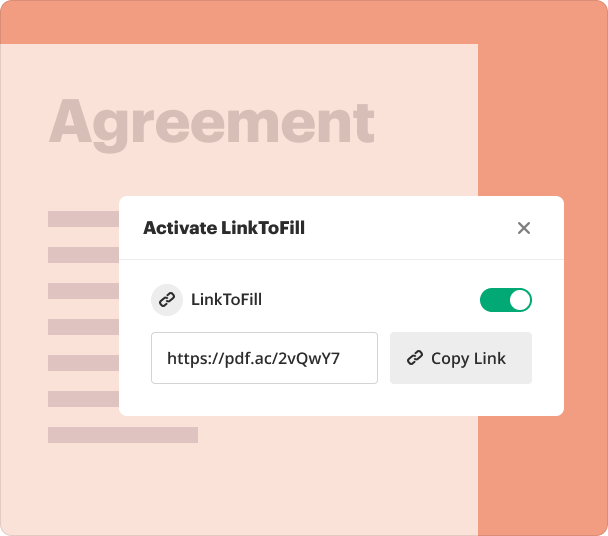
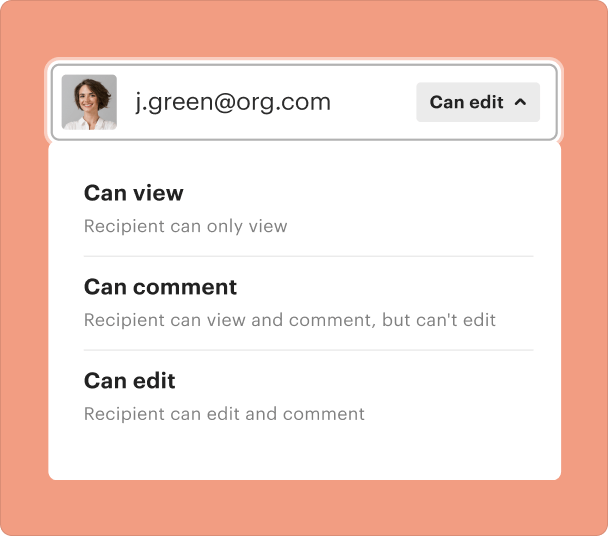
Sharing results and monitoring responses after you create a consent form
Sharing forms with potential participants is a breeze through pdfFiller. Users can track who has viewed, completed, or signed the document, ensuring transparency and accountability.
-
Create unique links for sharing your forms.
-
Access analytics to see form engagement.
-
Set reminders for participants who haven’t completed their forms.
-
View submission status in real time.
Exporting collected data once you create a consent form
After participants submit their consent forms, pdfFiller ensures that data handling is seamless. Users can easily export collected information for further analysis or record keeping.
-
Select the form and choose the 'Download' option.
-
Choose file format (CSV, Excel, etc.) for exporting.
-
Specify date range if needed for specific submissions.
-
Review and confirm the export.
Where and why businesses use a Clinical Trial Consent Form Creator
Several industries benefit from a Clinical Trial Consent Form Creator, primarily within healthcare, pharmaceuticals, and research. These sectors rely on systematic methods to document participant consent, ensuring adherence to ethical standards.
-
Pharmaceutical companies for clinical research.
-
CROs (Contract Research Organizations) handling multiple trials.
-
Academic institutions conducting research.
-
Healthcare providers seeking informed consent for treatments.
Conclusion
In conclusion, using pdfFiller’s Clinical Trial Consent Form Creator not only simplifies the process of creating essential documentation but also enhances compliance and accuracy throughout the clinical trial process. This powerful tool enables professionals to design, share, and analyze consent forms efficiently, ensuring that patient rights are upheld and that research adheres to legal standards.
-
Create a consent form that meets regulatory requirements.
-
Utilize collaborative features to enhance team productivity.
-
Track and analyze responses for better management.
How to create a PDF form
Who needs this?
The all-in-one PDF form creator, editor, and eSignature solution
Handle all your docs in one place
Keep data secure
Share and collaborate
pdfFiller scores top ratings on review platforms