Build PDF forms with pdfFiller’s Clinical Trial Report Form Creator
What is a Clinical Trial Report Form Creator?
The Clinical Trial Report Form Creator is a tool designed to streamline the process of creating, managing, and analyzing clinical trial documentation. This innovative tool allows users to build interactive PDF forms that cater specifically to the requirements of clinical trials, ensuring that all necessary data is collected efficiently and accurately.
How does the Clinical Trial Report Form Creator change document preparation?
Utilizing a Clinical Trial Report Form Creator can significantly reduce the time and effort needed in document preparation. By allowing users to create tailored forms and enforce data validation rules, this tool ensures that data collection is systematic and results are reliable. It enables faster adjustments to forms, which is critical in the ever-evolving landscape of clinical trials.
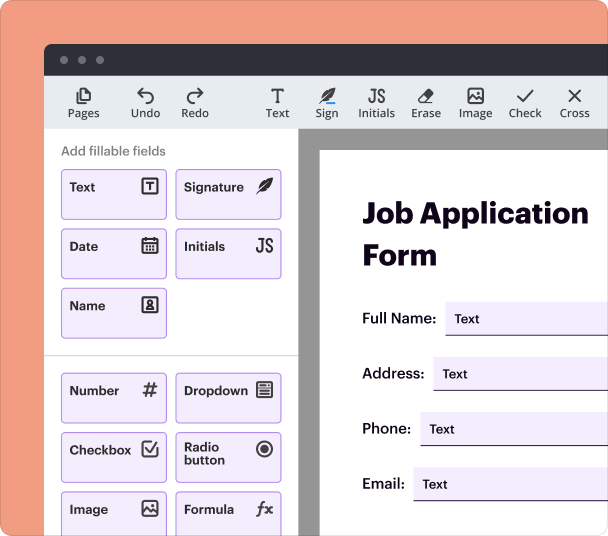
Steps to add fields when you create a Clinical Trial Report Form
To add interactive fields in your PDF form, follow these steps:
-
Open the Clinical Trial Report Form Creator in pdfFiller.
-
Select 'Add Field' from the toolbar.
-
Choose the type of field (e.g., text, checkbox, date) to include.
-
Click the desired location in the document to place the field.
-
Adjust the properties, such as field name and validation rules.
Setting validation and data rules as you create a Clinical Trial Report Form
Applying data rules ensures the integrity of the information collected. This is especially important in clinical trials where accurate data is crucial for compliance and safety.
-
Define mandatory fields to ensure critical information is captured.
-
Set specific data formats (e.g., dates in DD/MM/YYYY) for consistency.
-
Implement conditional logic for fields based on previous answers.
Going from blank page to finished form while you create a Clinical Trial Report Form
Creating a complete form from scratch is an efficient process with pdfFiller's tool. Start with a blank PDF or upload an existing document and build your form step-by-step.
-
Choose to start with a blank PDF or upload a document.
-
Utilize templates for common clinical report formats.
-
Add interactive fields as needed and adjust formatting.
Organizing and revising templates when you manage forms
Managing templates is as essential as creating them. pdfFiller allows for organized storage of your forms and quick revisions.
-
Store templates in clearly labeled folders.
-
Make revisions easily using the 'Edit' function.
-
Version control helps track changes and preserve earlier forms.
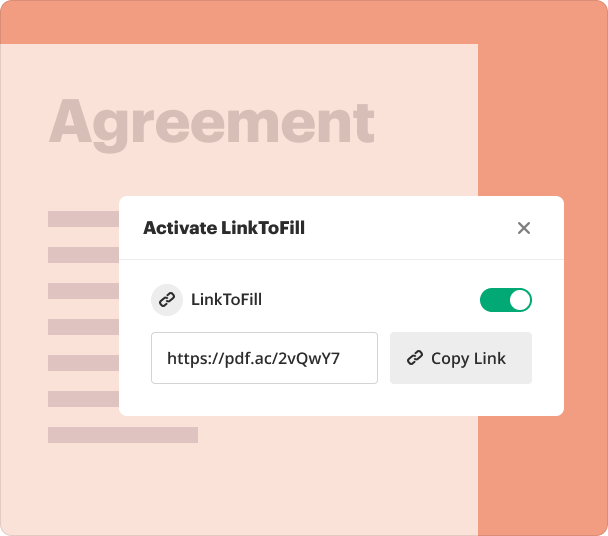
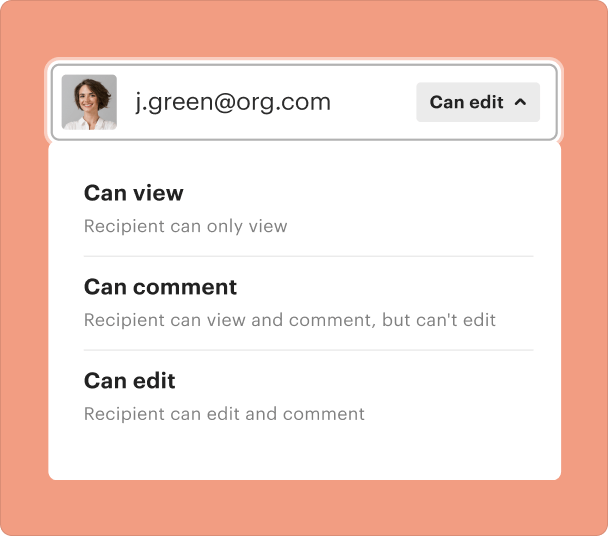
Sharing results and monitoring responses after you create a Clinical Trial Report Form
Once your form is ready, sharing and tracking responses is seamless with pdfFiller. This ensures stakeholders can access forms and results in real-time.
-
Share forms via email or direct link.
-
Monitor who has viewed or submitted the form.
-
Facilitate discussions and collaborations in real time.
Exporting collected data once you complete the form creation process
After collecting responses, exporting data for analysis is a straightforward process. pdfFiller enables seamless data integration with your preferred software tools.
-
Export data in various formats such as CSV, Excel, or PDF.
-
Integrate with research databases for efficient data management.
-
Use analytics tools for deeper insights into collected data.
Where and why businesses use a Clinical Trial Report Form Creator
Various industries, especially healthcare and pharmaceuticals, benefit significantly from a Clinical Trial Report Form Creator. These tools empower businesses to maintain regulatory compliance and facilitate comprehensive data collection.
Conclusion
The Clinical Trial Report Form Creator from pdfFiller transforms how clinical documentation is prepared and managed. By enabling the creation of specific and validated forms, it promotes efficiency and accuracy, vital for successful trials.
How to create a PDF form
Who needs this?
The all-in-one PDF form creator, editor, and eSignature solution
Handle all your docs in one place
Keep data secure
Share and collaborate
pdfFiller scores top ratings on review platforms