Build PDF forms with pdfFiller’s Dementia Research Consent Form Builder
What is a Dementia Research Consent Form Builder?
A Dementia Research Consent Form Builder is a specialized tool designed to help researchers easily create, customize, and manage consent forms related to dementia studies. This platform allows for the creation of professional-grade PDF forms that can capture essential information while ensuring compliance with regulatory requirements.
-
Easily customizable templates tailored for dementia research.
-
User-friendly interface accessible to individuals and teams.
-
Seamless integration with other tools and document formats.
-
Cloud-based access ensuring availability from any device.
-
Secure data handling compliant with HIPAA and other regulations.
How does a Dementia Research Consent Form Builder change document preparation?
Traditionally, preparing consent forms requires a lot of manual efforts with formatting, printing, and collecting signatures. A Dementia Research Consent Form Builder streamlines this process, allowing researchers to create and manage their documents digitally. This reduces time and effort, enhances accuracy, and ensures consistency across forms.
-
Simplifies the creation of multiple consent forms tailored for specific studies.
-
Facilitates collaboration among research teams working remotely.
-
Allows for real-time updates and revisions, maintaining relevance.
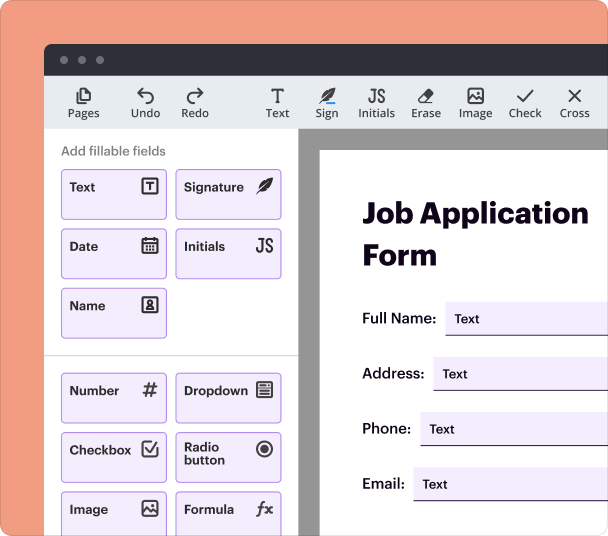
Steps to add interactive fields when using the Dementia Research Consent Form Builder
Adding interactive fields enhances the usability and functionality of your consent forms. The pdfFiller platform streamlines this by offering tools to introduce various input types, making it easy to gather the required information.
-
Open your PDF template within pdfFiller.
-
Select ‘Add Fields’ from the toolbar.
-
Choose the type of field you want to add (text box, checkbox, signature, etc.).
-
Drag and drop the selected field to the desired position on the form.
-
Customize field settings, including required input and formats.
Setting validation and data rules within the Dementia Research Consent Form Builder
Implementing data validation rules ensures that the information collected from participants is accurate and meets the necessary requirements. This feature helps prevent errors during data collection, essential for maintaining research integrity.
-
Select a field and access its properties for editing.
-
Enable validation options, such as character limits or data formats.
-
Set conditions for required fields to enhance completeness.
Going from blank page to finished form while using the Dementia Research Consent Form Builder
Creating a complete form from scratch can be daunting, but with pdfFiller's step-by-step tools, users can efficiently design their consent forms. The software also provides a library of pre-existing templates that can be customized, accelerating the development process.
-
Start with a blank form or clone an existing template to modify.
-
Outline the sections that will be included in the consent form.
-
Fill in all text areas and create the necessary interactive fields.
-
Review the form for accuracy and compliance with legal standards.
-
Save and export the completed form for distribution.
Organizing and revising templates when using the Dementia Research Consent Form Builder
Templates offer a foundational starting point for researchers, but maintaining and updating them is crucial as study requirements change. pdfFiller enables easy organization and modification of these templates to ensure relevance and accuracy.
-
Access the template library within your pdfFiller account.
-
Select templates needing updates or revisions.
-
Make changes directly within the platform and save updates.
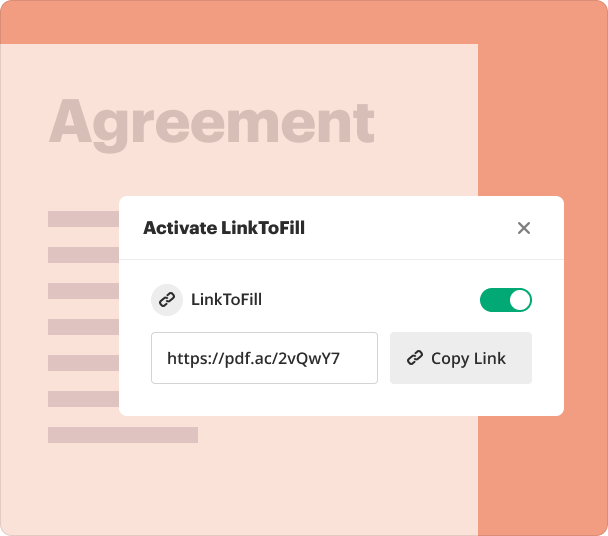
How can you share results and monitor responses after using the Dementia Research Consent Form Builder?
Once your consent forms are completed and distributed, it’s essential to track participation and responses. pdfFiller offers functionalities to monitor form completion and gather feedback effectively.
-
Share completed forms via direct links or email invitations.
-
Utilize tracking features to monitor who has completed the form.
-
Receive notifications for submissions and responses.
Exporting collected data once you use the Dementia Research Consent Form Builder
After form responses are collected, exporting data is key for analysis and record-keeping. pdfFiller ensures you can easily extract this information in various formats.
-
Access the responses summary within your pdfFiller account.
-
Select the export option that fits your needs (CSV, XLSX, PDF).
-
Download the exported data for further analysis or reporting.
Where and why do businesses use the Dementia Research Consent Form Builder?
Many healthcare organizations, academic institutions, and clinical research facilities utilize the Dementia Research Consent Form Builder to streamline their documentation processes. This tool is particularly valuable for facilitating collaboration among teams and ensuring compliance with ethical standards.
-
In clinical trials to inform and consent participants.
-
In research settings to maintain structured documentation.
-
Across healthcare facilities to standardize consent processes.
Conclusion
Utilizing the Dementia Research Consent Form Builder on pdfFiller significantly simplifies the often complex process of preparing consent documentation. With its interactive features, secure cloud capabilities, and comprehensive management tools, researchers can focus more on their studies rather than paperwork. By choosing pdfFiller, you'll not only enhance your efficiency but also ensure meticulous handling of all forms related to dementia research.
How to create a PDF form
Who needs this?
The all-in-one PDF form creator, editor, and eSignature solution
Handle all your docs in one place
Keep data secure
Share and collaborate
pdfFiller scores top ratings on review platforms