H Electronegativity
What is h electronegativity?
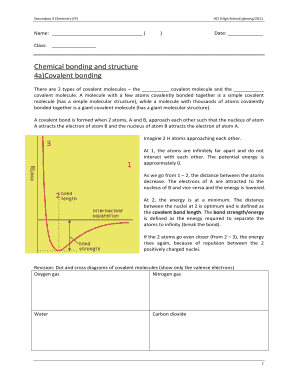
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. It helps in determining the polarity of a bond and the distribution of charge in a molecule. The concept of electronegativity was introduced by Linus Pauling, who assigned numerical values to elements based on their affinity for electrons.
What are the types of h electronegativity?
There are several scales available to measure electronegativity, but the two most commonly used are the Pauling scale and the Mulliken scale. The Pauling scale gives electronegativity values ranging from 0.7 for cesium to 4.0 for fluorine, while the Mulliken scale defines electronegativity as the average of the ionization potential and electron affinity.
How to complete h electronegativity
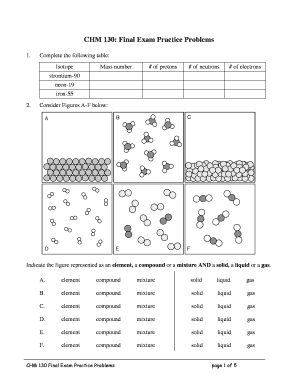
To complete the electronegativity of an atom, follow these steps:
By following these steps, you can effectively determine the electronegativity of an atom and gain insights into chemical bonding and molecular properties.