Get the free Summary - accessdata fda
Show details
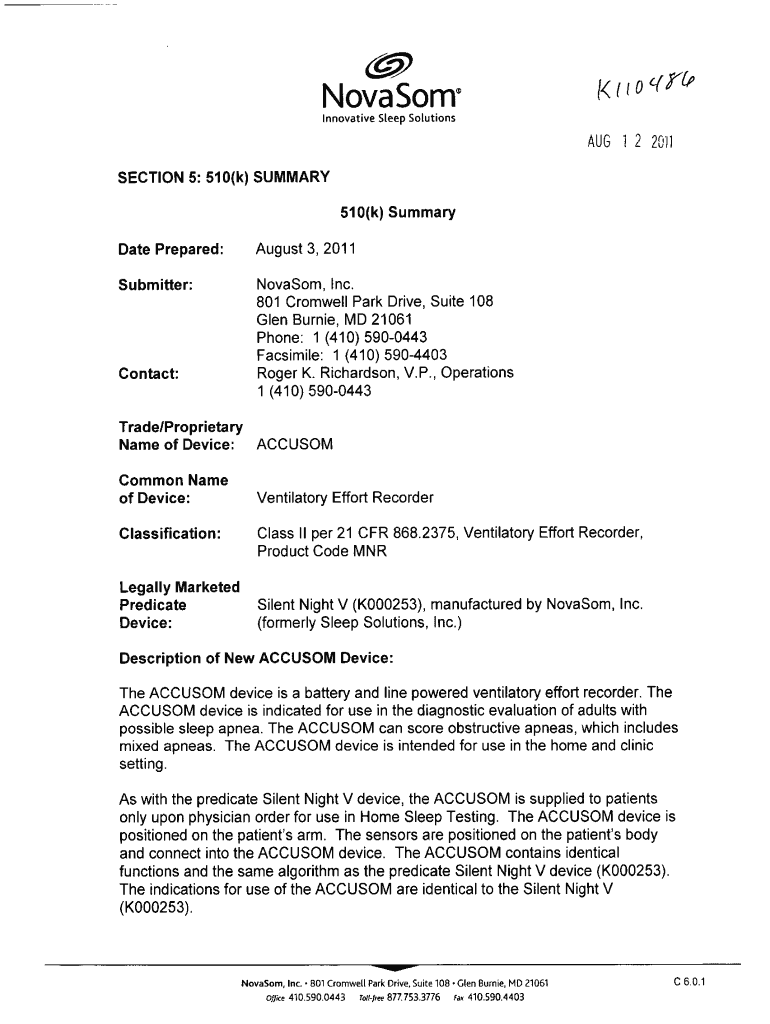
Nova Some Innovative Steep Solutions AUG 12 20fl SECTION 5: 51 0(k) SUMMARY 510(k) Summary Date Prepared: August 3, 2011, Submitter: Novas on, Inc. 801 Cromwell Park Drive, Suite 108 Glen Burnie,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign summary - accessdata fda

Edit your summary - accessdata fda form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your summary - accessdata fda form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing summary - accessdata fda online
To use the services of a skilled PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit summary - accessdata fda. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
Dealing with documents is always simple with pdfFiller. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out summary - accessdata fda

How to fill out summary - accessdata FDA:
01
Begin by gathering all the necessary information related to the accessdata FDA. This may include data about the product or service being accessed, any relevant documents or reports, and contact information for the FDA.
02
Start by clearly stating the purpose of the summary. This will help provide context and guide the reader in understanding the information being presented.
03
Provide a brief overview of the accessdata FDA process. Explain any steps or requirements that need to be followed in order to successfully complete the accessdata FDA.
04
Break down the summary into sections or subsections, depending on the complexity and length of the information. This will help organize the content and make it easier for the reader to navigate.
05
Include relevant details about the accessdata FDA, such as any specific forms that need to be filled out, deadlines that need to be met, and any supporting documentation that needs to be submitted.
06
Clearly explain any terminology or jargon that may be used in the accessdata FDA process. This will ensure that the reader can fully understand the information being presented.
07
Provide guidance on how to complete each section of the summary. This may involve explaining the purpose of each section, providing examples, or offering tips and tricks for successfully completing that section.
Who needs summary - accessdata FDA:
01
Regulatory professionals: Individuals working in the regulatory field who are responsible for submitting accessdata FDA documents on behalf of their organization. They need a summary in order to understand the key information and requirements for successful completion.
02
Researchers and developers: Those involved in the research and development of products or services regulated by the FDA. They may need a summary to ensure they provide accurate and complete information when accessing the FDA's data.
03
Compliance officers: Individuals responsible for ensuring that their organization meets all FDA regulations and requirements. They may need a summary to understand the accessdata FDA process and to ensure that their organization is in compliance.
In conclusion, filling out the summary for accessdata FDA requires gathering relevant information, clearly stating the purpose, providing an overview of the process, organizing the content, explaining details, and offering guidance. This summary is needed by regulatory professionals, researchers and developers, and compliance officers to successfully navigate the accessdata FDA process.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my summary - accessdata fda in Gmail?
summary - accessdata fda and other documents can be changed, filled out, and signed right in your Gmail inbox. You can use pdfFiller's add-on to do this, as well as other things. When you go to Google Workspace, you can find pdfFiller for Gmail. You should use the time you spend dealing with your documents and eSignatures for more important things, like going to the gym or going to the dentist.
How do I complete summary - accessdata fda on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your summary - accessdata fda from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
How do I complete summary - accessdata fda on an Android device?
Use the pdfFiller app for Android to finish your summary - accessdata fda. The application lets you do all the things you need to do with documents, like add, edit, and remove text, sign, annotate, and more. There is nothing else you need except your smartphone and an internet connection to do this.
What is summary - accessdata fda?
Summary - accessdata fda is a summary report required by the FDA to provide an overview of important information regarding a regulated product.
Who is required to file summary - accessdata fda?
Manufacturers, distributors, and importers of regulated products are required to file summary - accessdata fda.
How to fill out summary - accessdata fda?
The summary - accessdata fda can be filled out online through the FDA's electronic submission system or by submitting a paper copy via mail.
What is the purpose of summary - accessdata fda?
The purpose of summary - accessdata fda is to ensure that important information about regulated products is readily available to the FDA and other regulatory agencies.
What information must be reported on summary - accessdata fda?
The summary - accessdata fda must include information such as product name, manufacturer information, intended use, adverse events, and any recalls or safety alerts.
Fill out your summary - accessdata fda online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Summary - Accessdata Fda is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















