Get the free Paragraph IV Patent Certifications
Show details
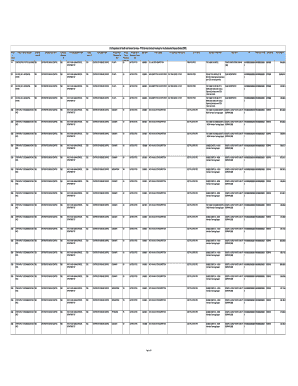
Paragraph IV Patent Certifications September 13, 2017DRUG NAMESAKE FORMSTRENGTHRLDDATE OF SUBMISSIONAbacavir SulfateTablets300 mgZiagen1/28/2009AbacavirOral Solution20 mg/mLZiagen12/27/2012Abacavir
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign paragraph iv patent certifications

Edit your paragraph iv patent certifications form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your paragraph iv patent certifications form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit paragraph iv patent certifications online
Use the instructions below to start using our professional PDF editor:
1
Log in to account. Click Start Free Trial and register a profile if you don't have one.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit paragraph iv patent certifications. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
It's easier to work with documents with pdfFiller than you could have believed. You can sign up for an account to see for yourself.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out paragraph iv patent certifications

How to fill out paragraph IV patent certifications:
01
Start by carefully reading and understanding the requirements and instructions for filling out the paragraph IV patent certification form. Familiarize yourself with the specific details and information that need to be provided.
02
Begin by identifying the patent at issue. This includes providing the patent number, the expiration date of the patent, and the owner or assignee of the patent. Ensure that all the patent details are accurate and up-to-date.
03
Next, specify the drug for which the certification is being made. Provide the drug's generic name, brand name if applicable, and its application or NDA number. Make sure to accurately input all the necessary drug details.
04
Choose the type of certification you are making. There are four different types of paragraph IV certifications: (a) No patent information required, (b) Patent expired, (c) Patent will expire on a certain date, or (d) Patent is invalid or won't be infringed upon.
05
Depending on the type of certification chosen, additional information may be required. If the patent has already expired, provide the date of expiration. If the patent will expire on a specific date, indicate that date.
06
If selecting the certification that the patent is invalid or won't be infringed upon, provide a statement explaining the legal or factual basis for this assertion. Make sure to provide a clear and concise explanation.
07
Review the filled-out form for accuracy, ensuring that all the information provided is correct and complete. Make any necessary revisions or corrections before submitting the form.
Who needs paragraph IV patent certifications:
01
Generic drug manufacturers: Generic drug manufacturers must submit paragraph IV patent certifications when they are seeking approval from the FDA to market a generic version of a brand-name drug before the expiration of the related patents.
02
Legal counsel: Attorneys representing generic drug manufacturers typically handle the preparation and submission of paragraph IV patent certifications on behalf of their clients. They ensure that the certifications are accurate and comply with all relevant laws and regulations.
03
Brand-name drug manufacturers: Paragraph IV patent certifications can also be relevant for brand-name drug manufacturers who receive notifications of paragraph IV certifications from generic drug manufacturers. Brand-name manufacturers may need to respond to such certifications by initiating patent infringement litigation.
Note: It is advised to consult with a legal expert or patent attorney to ensure compliance with all legal requirements related to paragraph IV patent certifications.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify paragraph iv patent certifications without leaving Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your paragraph iv patent certifications into a dynamic fillable form that can be managed and signed using any internet-connected device.
How can I send paragraph iv patent certifications for eSignature?
Once you are ready to share your paragraph iv patent certifications, you can easily send it to others and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail, or notarize it online. You can do all of this without ever leaving your account.
How do I fill out paragraph iv patent certifications on an Android device?
Use the pdfFiller app for Android to finish your paragraph iv patent certifications. The application lets you do all the things you need to do with documents, like add, edit, and remove text, sign, annotate, and more. There is nothing else you need except your smartphone and an internet connection to do this.
What is paragraph iv patent certifications?
Paragraph IV patent certifications are a legal statement made by a company that it intends to market a generic version of a patented drug before the patent for the original drug expires.
Who is required to file paragraph iv patent certifications?
Any company planning to market a generic version of a patented drug before the patent expires is required to file paragraph IV patent certifications.
How to fill out paragraph iv patent certifications?
Paragraph IV patent certifications are typically submitted to the US Food and Drug Administration (FDA) as part of the Abbreviated New Drug Application (ANDA) process.
What is the purpose of paragraph iv patent certifications?
The purpose of paragraph IV patent certifications is to challenge the validity of the patents held by the company that manufactures the original drug, in order to gain approval to market a generic version.
What information must be reported on paragraph iv patent certifications?
Paragraph IV patent certifications must include details of the patents that the company believes are invalid or not infringed by their generic product.
Fill out your paragraph iv patent certifications online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Paragraph Iv Patent Certifications is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.