Get the free 510K Notification Supplement
Show details
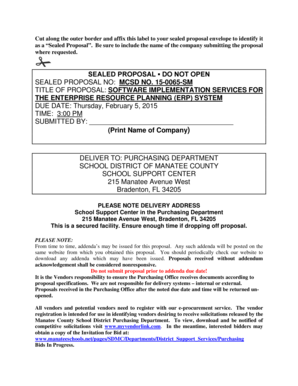
510K Notification Supplement K101893 S/1ior December 31, 2011 510k SUMMARY (as per 21 CFIX807.92(b) (1) FE5 1720GENERAL INFORMATION Device Generic Name: Trade Name:Nexus Nexus Nexus NexusInfrared
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign 510k notification supplement

Edit your 510k notification supplement form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your 510k notification supplement form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing 510k notification supplement online
To use the services of a skilled PDF editor, follow these steps below:
1
Log in to account. Click Start Free Trial and sign up a profile if you don't have one yet.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit 510k notification supplement. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out 510k notification supplement

How to fill out 510k notification supplement:
01
Begin by reviewing the instructions provided with the 510k notification supplement. It is important to familiarize yourself with the specific requirements and details mentioned in the instructions.
02
Collect all the necessary information and documentation required for the notification supplement. This may include data on the device, its intended use, any previous submissions or clearances, and any changes or modifications being made.
03
Complete the administrative section of the notification supplement. This typically involves providing general information about the manufacturer, contact details, and a brief description of the device.
04
Provide a comprehensive description of the device in the technical section. This should include details about its intended use, design specifications, components, and any relevant performance testing or clinical data.
05
If any changes have been made to the device or its manufacturing process, include this information in the appropriate section. Clearly explain the nature of the changes and their potential impact on the safety and performance of the device.
06
Include a well-structured and thorough risk analysis that addresses any potential hazards associated with the device. This should cover aspects such as design, materials, manufacturing, and intended use.
07
Provide a summary of any validation testing conducted to demonstrate the safety and effectiveness of the device. Include details of the testing methods, results, and any necessary explanations or justifications.
08
If applicable, include any labeling changes or updates that have been made to the device. This may involve providing revised instructions for use, warnings, or precautions.
09
Review and double-check all the information provided in the notification supplement to ensure accuracy and completeness. Make sure that all required sections have been properly addressed and that all necessary attachments or supporting documentation have been included.
10
Finally, submit the completed 510k notification supplement to the appropriate regulatory authority or agency. Follow their specific submission guidelines and ensure that all required forms and fees are included.
Who needs 510k notification supplement?
01
Medical device manufacturers who intend to introduce a new medical device into the US market that requires premarket notification, as outlined by the Food and Drug Administration (FDA), need the 510k notification supplement.
02
Manufacturers who have made any significant changes or modifications to an existing FDA-cleared medical device may also need to submit a 510k notification supplement to provide the necessary documentation and information about the changes made.
03
Additionally, manufacturers who have previously received a 510k clearance for a similar device but are now introducing a different model or design may also be required to submit a 510k notification supplement. This is to ensure that the new device complies with the necessary regulations and requirements for market entry.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is 510k notification supplement?
A 510k notification supplement is a submission to the FDA that provides additional information or data related to a previously cleared 510k medical device.
Who is required to file 510k notification supplement?
Manufacturers of medical devices who have already obtained 510k clearance and need to make changes or provide additional information are required to file a 510k notification supplement.
How to fill out 510k notification supplement?
To fill out a 510k notification supplement, manufacturers must provide detailed information about the changes or additional data being submitted, along with any supporting documentation.
What is the purpose of 510k notification supplement?
The purpose of a 510k notification supplement is to inform the FDA of changes or additions to a previously cleared medical device to ensure continued safety and effectiveness.
What information must be reported on 510k notification supplement?
Information required on a 510k notification supplement includes details of the changes being made, any new data or testing results, and how these changes impact the safety and effectiveness of the device.
How can I send 510k notification supplement to be eSigned by others?
When you're ready to share your 510k notification supplement, you can send it to other people and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail. You can also notarize your PDF on the web. You don't have to leave your account to do this.
How do I fill out the 510k notification supplement form on my smartphone?
You can quickly make and fill out legal forms with the help of the pdfFiller app on your phone. Complete and sign 510k notification supplement and other documents on your mobile device using the application. If you want to learn more about how the PDF editor works, go to pdfFiller.com.
How do I complete 510k notification supplement on an Android device?
Complete 510k notification supplement and other documents on your Android device with the pdfFiller app. The software allows you to modify information, eSign, annotate, and share files. You may view your papers from anywhere with an internet connection.
Fill out your 510k notification supplement online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

510k Notification Supplement is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.