Get the free Genentech Inc 483 for 09/2011 - fda
Show details
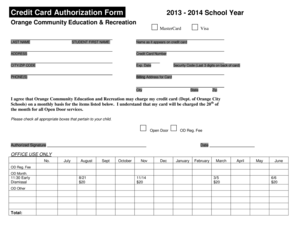
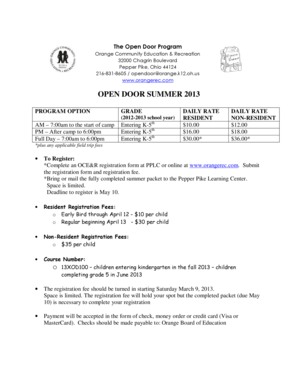
DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG Administration DISTRICT ADDRESS AND P ION ENE NUMBER DATE(S)Of INSPECTION 1431 Harbor Bay Parkway Alameda, CA 94502-7070 (520) 337-6700 Fax (510)
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign genentech inc 483 for

Edit your genentech inc 483 for form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your genentech inc 483 for form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit genentech inc 483 for online
Follow the steps down below to benefit from the PDF editor's expertise:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit genentech inc 483 for. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out genentech inc 483 for

How to fill out the Genentech Inc 483 form:
01
Carefully read the instructions provided on the Genentech Inc 483 form. Make sure you understand all the requirements and information needed.
02
Begin by entering your personal details accurately. This typically includes your name, contact information, and any relevant identification numbers.
03
Provide the necessary information about the incident or issue that led to the need for the Genentech Inc 483 form. Include specific dates, locations, and any parties involved.
04
Describe the details of the incident or issue in a clear and concise manner. Provide as much relevant information as possible, and be honest and accurate in your account.
05
If applicable, attach any supporting documents or evidence to provide further context or evidence of the incident or issue.
06
Review all the information you have entered to ensure accuracy and completeness. Double-check for any errors or missing information.
07
Sign and date the form to confirm that all the provided information is true and accurate to the best of your knowledge.
08
Submit the completed Genentech Inc 483 form to the designated recipient or department as indicated in the instructions.
Who needs the Genentech Inc 483 form?
01
Individuals or employees who have witnessed or experienced an incident related to the operations, protocols, or practices of the Genentech Inc organization.
02
Regulatory bodies or government agencies responsible for conducting inspections or investigations into Genentech Inc's compliance with regulations and standards.
03
Legal authorities or colleagues who need to document and report any violations, non-compliance, or potential risks associated with Genentech Inc's activities.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I fill out the genentech inc 483 for form on my smartphone?
You can quickly make and fill out legal forms with the help of the pdfFiller app on your phone. Complete and sign genentech inc 483 for and other documents on your mobile device using the application. If you want to learn more about how the PDF editor works, go to pdfFiller.com.
Can I edit genentech inc 483 for on an iOS device?
You certainly can. You can quickly edit, distribute, and sign genentech inc 483 for on your iOS device with the pdfFiller mobile app. Purchase it from the Apple Store and install it in seconds. The program is free, but in order to purchase a subscription or activate a free trial, you must first establish an account.
How do I edit genentech inc 483 for on an Android device?
With the pdfFiller mobile app for Android, you may make modifications to PDF files such as genentech inc 483 for. Documents may be edited, signed, and sent directly from your mobile device. Install the app and you'll be able to manage your documents from anywhere.
What is genentech inc 483 for?
Genentech Inc 483 is a form used by the U.S. Food and Drug Administration (FDA) to document and communicate inspection findings and violations of regulations during inspections of Genentech Inc facilities.
Who is required to file genentech inc 483 for?
Genentech Inc is required to file the Genentech Inc 483 form when the FDA finds any significant violations or deviations from regulatory requirements during their inspections of Genentech Inc facilities.
How to fill out genentech inc 483 for?
To fill out the Genentech Inc 483 form, specific details of the inspection findings, violations, and deviations must be accurately documented. The form must also include the relevant dates, locations, and any corrective actions taken or planned by Genentech Inc.
What is the purpose of genentech inc 483 for?
The purpose of the Genentech Inc 483 form is to inform Genentech Inc management and FDA about inspections findings and violations. It serves as a formal notice for Genentech Inc to address the issues identified and take necessary corrective actions to ensure compliance with regulations.
What information must be reported on genentech inc 483 for?
The Genentech Inc 483 form should report the inspection findings, violations, and deviations from regulatory requirements. It must include details such as the specific regulations violated, dates, locations, observations made by the FDA inspector, and any corrective actions taken or planned.
Fill out your genentech inc 483 for online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Genentech Inc 483 For is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.