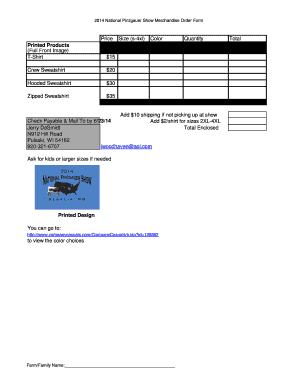
Get the free Letter to FDA Regarding GE Food Safety Regulations - fda
Show details
A letter addressed to the FDA expressing concerns and requests regarding the regulation, labeling, and safety testing of genetically engineered foods.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign letter to fda regarding

Edit your letter to fda regarding form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your letter to fda regarding form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing letter to fda regarding online
To use our professional PDF editor, follow these steps:
1
Log in to your account. Start Free Trial and sign up a profile if you don't have one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit letter to fda regarding. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out letter to fda regarding

How to fill out Letter to FDA Regarding GE Food Safety Regulations
01
Start with your contact information at the top right corner.
02
Include the date below your contact information.
03
Write the FDA's contact information on the left side, below the date.
04
Begin the letter with an appropriate salutation, such as 'Dear FDA Officials'.
05
Introduce yourself and your organization, if applicable.
06
Clearly state the purpose of your letter regarding GE food safety regulations.
07
Outline your concerns or comments about the regulations in a structured manner.
08
Provide any relevant data or examples to support your position.
09
Conclude with a summary of your main points and a call to action or request for a response.
10
End the letter with a polite closing, such as 'Sincerely', followed by your name and signature.
Who needs Letter to FDA Regarding GE Food Safety Regulations?
01
Individuals or organizations concerned about the safety of genetically engineered food.
02
Farmers and agricultural producers involved in growing or selling GE crops.
03
Consumer advocacy groups focused on food safety and transparency.
04
Policy makers and legislators interested in food regulation.
Fill
form
: Try Risk Free






People Also Ask about
What is a letter of guarantee for food safety?
(Name of person giving the guaranty or undertaking) hereby guarantees that no article listed herein is adulterated or misbranded within the meaning of the Federal Food, Drug, and Cosmetic Act, or is an article which may not, under the provisions of section 404, 505, or 512 of the act, be introduced into interstate
What are examples of FDA violations?
Import Refusals adulterated, meaning the product is contaminated, is not safe, or does not otherwise meet applicable standards; misbranded, meaning the labels contain false or misleading information; an unapproved new drug; forbidden or restricted for sale.
How do I contact FDA Food Safety?
If you wish to speak directly to a person about your complaint or adverse event, please call the Food and Cosmetic Information Center at 1‐888‐SAFEFOOD (1‐888‐723‐3366) Monday - Friday 10:00 AM - 4:00 PM ET – Closed Thursdays from 12:30 PM - 1:30 PM ET and Federal holidays.
How do I make a complaint to the FDA?
Please direct concerns to the appropriate FDA center by visiting our SmartHub webpage, which will guide you to the appropriate webform or phone number. If you are not able to use the SmartHub, you may also call 1-888-INFO-FDA and follow the prompts to report a problem.
How do I complain to the FDA about food safety?
Phone the FDA Main Emergency Number at 888-SAFEFOOD (888-723-3366) or report online through the FDA Safety Reporting Portal. For cosmetics use the MedWatch Online Voluntary Reporting Form. Phone a CVM Complaint Coordinator at 240-402-3876 or report online through the FDA Safety Reporting Portal.
What are 5 things the FDA regulates?
The Food and Drug Administration (FDA) is responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, our nation's food supply, cosmetics, and products that emit radiation.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Letter to FDA Regarding GE Food Safety Regulations?
The Letter to FDA Regarding GE Food Safety Regulations is a formal communication submitted to the U.S. Food and Drug Administration to address the safety and regulatory framework surrounding genetically engineered (GE) foods.
Who is required to file Letter to FDA Regarding GE Food Safety Regulations?
Producers, manufacturers, or stakeholders involved in the development, production, or commercialization of genetically engineered foods are required to file this letter.
How to fill out Letter to FDA Regarding GE Food Safety Regulations?
To fill out the letter, stakeholders must provide relevant information including the identity of the GE organism, the intended use, safety assessments, and any additional data required by the FDA.
What is the purpose of Letter to FDA Regarding GE Food Safety Regulations?
The purpose of the letter is to ensure transparency and safety of GE foods, allowing the FDA to evaluate potential health impacts and ensure that regulatory standards are met.
What information must be reported on Letter to FDA Regarding GE Food Safety Regulations?
This letter must report information such as the genetic modifications made, potential allergenicity, nutritional profiles, and results from safety assessments conducted.
Fill out your letter to fda regarding online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Letter To Fda Regarding is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.