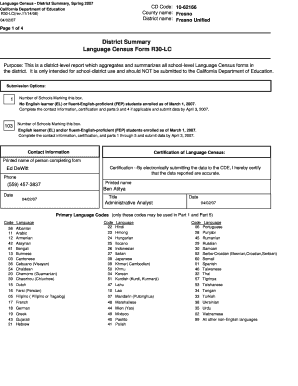
Get the free Draft Guidance for Industry: Questions and Answers Regarding the Labeling of Dietary...
Show details
This document provides guidance for manufacturers, packers, and distributors regarding the labeling requirements for dietary supplements under the Dietary Supplement and Nonprescription Drug Consumer
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign draft guidance for industry

Edit your draft guidance for industry form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your draft guidance for industry form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing draft guidance for industry online
To use our professional PDF editor, follow these steps:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit draft guidance for industry. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out draft guidance for industry

How to fill out Draft Guidance for Industry: Questions and Answers Regarding the Labeling of Dietary Supplements as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act
01
Read the Draft Guidance document thoroughly to understand its structure and requirements.
02
Identify the specific sections that pertain to labeling of dietary supplements.
03
Gather all necessary product information including ingredients, claims, and any relevant scientific data.
04
Follow the formatting guidelines presented in the Draft Guidance for presenting your labeling information.
05
Ensure clarity and accuracy in the information provided, avoiding misleading statements.
06
Cite any studies or sources that support the claims made on the label as required by the Guidance.
07
Review the completed labeling for compliance with both the Draft Guidance and the Dietary Supplement and Nonprescription Drug Consumer Protection Act.
08
Submit the labeling information as instructed in the Draft Guidance.
Who needs Draft Guidance for Industry: Questions and Answers Regarding the Labeling of Dietary Supplements as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act?
01
Manufacturers of dietary supplements seeking to ensure compliance with labeling regulations.
02
Regulatory affairs professionals working in the dietary supplement industry.
03
Legal advisors in the dietary supplement sector who need to understand compliance requirements.
04
Quality assurance teams responsible for product labeling accuracy.
05
Industry stakeholders interested in the safety and efficacy of dietary supplement labels.
Fill
form
: Try Risk Free






People Also Ask about
What are the FDA labeling guidelines for supplements?
Dietary Supplement Labeling Must Include: Name of product. Net quantity of contents. Name and address of the manufacturer, packer, or distributor. Directions for use.
Are there regulations for supplements?
Dietary supplement marketing, manufacturing, labeling, and advertising are all covered by regulations enforced by FDA and the Federal Trade Commission.
Does the EPA regulate the labeling of dietary supplements?
The EPA regulates the labeling of dietary supplements. The FDA can recall a dietary supplements when there is evidence that it is harmful.
What are the regulations for supplement labeling?
Labelling requirements Food supplements must comply with general food labelling rules and display: portion of the product recommended for daily consumption. warning not to exceed the recommended daily dose. statement that food supplements should not be used as a substitute for a balanced diet.
Is an organization that provides some guidance for consumers when it comes to labeling dietary supplements?
Federal Regulation of Dietary Supplements FDA is the federal agency that oversees both supplements and medicines, but FDA regulations for dietary supplements are different from those for prescription or over-the-counter medicines. Medicines must be approved by FDA before they can be sold or marketed.
Which 21 CFR regulations should a company need to follow for dietary supplements?
The Dietary Supplement (DS) CGMP rule in 21 CFR part 111 (“the DS CGMP rule”) requires persons who manufacture, package, label, or hold a dietary supplement to establish and follow current good manufacturing practice to ensure the quality of the dietary supplement and to ensure that the dietary supplement is packaged
What are the FDA labeling requirements for supplements?
FDA regulations require dietary supplement labels to bear a product name and a statement that it is a "dietary supplement" or equivalent term replacing "dietary" with the name or type of dietary ingredient in the product (e.g., "iron supplement" or "herbal supplement"); the name and place of business of the
What is the dietary supplement and Nonprescription drug Consumer Protection Act?
Effective December 22, 2007, the Dietary Supplement and Nonprescription Drug Consumer Protection Act mandates that manufacturers, packers, and distributors of dietary supplements in the U.S. report information about serious adverse events associated with the use of these supplements to FDA.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Draft Guidance for Industry: Questions and Answers Regarding the Labeling of Dietary Supplements as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act?
The Draft Guidance for Industry provides clarification and answers to questions regarding the labeling requirements for dietary supplements as mandated by the Dietary Supplement and Nonprescription Drug Consumer Protection Act. It aims to assist manufacturers and marketers in complying with federal regulations.
Who is required to file Draft Guidance for Industry: Questions and Answers Regarding the Labeling of Dietary Supplements as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act?
Manufacturers, packers, and distributors of dietary supplements are required to adhere to the guidelines laid out in the Draft Guidance for Industry regarding the labeling of their products.
How to fill out Draft Guidance for Industry: Questions and Answers Regarding the Labeling of Dietary Supplements as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act?
Filing involves following the specific labeling requirements outlined in the guidance. This includes ensuring that labels are accurate, truthful, and not misleading, and that they contain all necessary information such as ingredients, serving sizes, and claims.
What is the purpose of Draft Guidance for Industry: Questions and Answers Regarding the Labeling of Dietary Supplements as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act?
The purpose of the Draft Guidance is to ensure consumer safety by promoting clear and accurate labeling of dietary supplements, thereby enabling consumers to make informed choices regarding their health products.
What information must be reported on Draft Guidance for Industry: Questions and Answers Regarding the Labeling of Dietary Supplements as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act?
The information required includes details about the product's identity, nutritional information, any health claims, ingredient list, and manufacturing details. Accurate reporting is essential for compliance with the law.
Fill out your draft guidance for industry online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Draft Guidance For Industry is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.