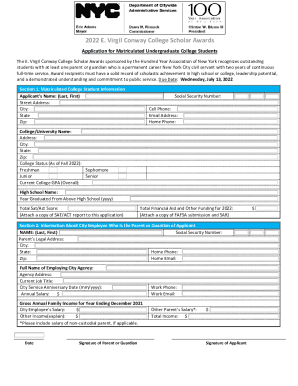
Get the free Inspection by Accredited Persons Program Under the Medical Device User Fee and Moder...
Show details
This document outlines the FDA's program for accrediting third parties to conduct inspections of manufacturers of class II or III medical devices under the Medical Device User Fee and Modernization
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign inspection by accredited persons

Edit your inspection by accredited persons form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your inspection by accredited persons form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing inspection by accredited persons online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit inspection by accredited persons. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out inspection by accredited persons

How to fill out Inspection by Accredited Persons Program Under the Medical Device User Fee and Modernization Act of 2002
01
Review the requirements of the Inspection by Accredited Persons Program outlined in the Medical Device User Fee and Modernization Act of 2002.
02
Ensure your facility meets the eligibility criteria for participation in the program.
03
Identify an accredited organization that can conduct the inspection.
04
Prepare for the inspection by ensuring all relevant documentation and records are organized and accessible.
05
Schedule the inspection with the accredited organization.
06
Conduct a pre-inspection meeting with the inspection team to clarify the process and expectations.
07
Participate in the inspection, addressing any questions or concerns raised by the inspectors.
08
After the inspection, review the findings and recommendations provided by the inspection team.
09
Implement any necessary corrective actions based on the inspection results.
10
Maintain records of the inspection and any follow-up actions taken.
Who needs Inspection by Accredited Persons Program Under the Medical Device User Fee and Modernization Act of 2002?
01
Manufacturers of medical devices who wish to participate in the Inspection by Accredited Persons Program.
02
Medical device firms looking to streamline their inspection processes under FDA regulations.
03
Businesses aiming to gain compliance with the Medical Device User Fee and Modernization Act of 2002.
Fill
form
: Try Risk Free






People Also Ask about
What are the 3 main types of inspections?
There are 3 inspection task types: preventive control inspections, sample collection, and commodity inspections. Preventive control inspection. Sample collection. Commodity inspection. The inspection process. 3.1 Determine the scope of the inspection. 3.2 Establish the team. 3.3 Review information.
What is the Medical Device User fee and Modernization Act 2002?
The Medical Device User Fee and Modernization Act of 2002 (MDUFMA), P.L 107-250, amends the Federal Food, Drug, and Cosmetic Act (FD&C Act) to provide FDA new responsibilities, resources, and challenges. MDUFMA was signed into law October 26, 2002.
What are the 4 types of FDA inspections?
The four different types of FDA inspections are pre-approval inspection, routine inspection, compliance follow-up inspection, and “for cause” inspection. Each is intended to help protect the public from unsafe products, but the focus and expectations of each type of inspection are different.
What is Section 206 of the medical device User Charge and Modernization Act Mdufma?
(Sec. 206) Allows labeling for prescription devices intended for use in health care facilities to be made available solely by electronic means, provided that a manufacturer furnishes a paper copy for free upon request by a health care facility.
What are the different types of FDA inspections?
There are Four Types of FDA Inspections, but They Don't Apply to Everyone. There are four types of FDA inspections for medical device manufacturers: pre-approval inspections, routine inspections, follow-up inspections, and for cause inspections.
What are the 4 types of quality inspection?
In quality control, there are 4 types of quality inspections, namely: Pre-Production Inspection, During Production Inspection, Pre-Shipment Inspection, and Container Loading/Unloading Inspections. Each of these types of inspection has its own purpose.
What are the 4 types of FDA enforcement actions?
Enforcement Actions (CBER) Warning Letters. Notice of Initiation of Disqualification Proceedings and Opportunity to Explain (NIDPOE) Letters. Untitled Letters. Administrative License Action Letters.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Inspection by Accredited Persons Program Under the Medical Device User Fee and Modernization Act of 2002?
The Inspection by Accredited Persons Program is a framework established under the Medical Device User Fee and Modernization Act of 2002 that allows certain accredited organizations to conduct inspections of medical device manufacturers on behalf of the FDA, thereby streamlining the regulatory process.
Who is required to file Inspection by Accredited Persons Program Under the Medical Device User Fee and Modernization Act of 2002?
Medical device manufacturers who are subject to premarket approval and those seeking to comply with FDA inspection requirements are required to participate in the Inspection by Accredited Persons Program.
How to fill out Inspection by Accredited Persons Program Under the Medical Device User Fee and Modernization Act of 2002?
To fill out the necessary documentation for the Inspection by Accredited Persons Program, manufacturers must provide detailed information about their facility, the devices being manufactured, and evidence of compliance with relevant standards, as specified by the accredited organization.
What is the purpose of Inspection by Accredited Persons Program Under the Medical Device User Fee and Modernization Act of 2002?
The purpose of the Inspection by Accredited Persons Program is to enhance the efficiency and effectiveness of the medical device regulatory process by utilizing accredited third-party organizations to conduct inspections, thus reducing the burden on FDA resources.
What information must be reported on Inspection by Accredited Persons Program Under the Medical Device User Fee and Modernization Act of 2002?
Manufacturers must report inspection outcomes, compliance statuses, any non-conformities identified during the inspection, and corrective actions taken. This information is critical for maintaining quality and safety standards in medical device manufacturing.
Fill out your inspection by accredited persons online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Inspection By Accredited Persons is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.