Get the free APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B - cdph ca
Show details
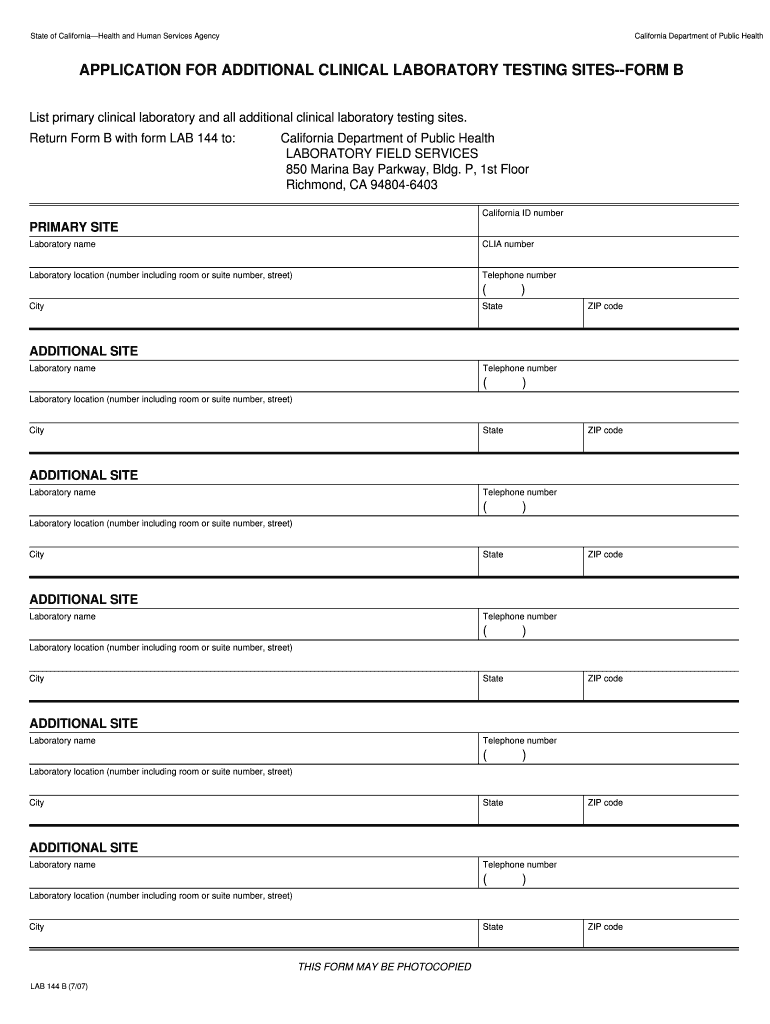
A form used for listing primary and additional clinical laboratory testing sites as part of the application process for laboratory testing in California.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign application for additional clinical

Edit your application for additional clinical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your application for additional clinical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit application for additional clinical online
Use the instructions below to start using our professional PDF editor:
1
Sign into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit application for additional clinical. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you could have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out application for additional clinical

How to fill out APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B
01
Begin by downloading the APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B from the official website.
02
Enter the name of the clinical laboratory and its address at the top of the form.
03
Fill in the contact information including phone number and email address.
04
Include the state license number and CLIA (Clinical Laboratory Improvement Amendments) number in the appropriate sections.
05
List all additional testing sites you wish to include, one per line, along with their respective addresses.
06
Provide a brief description of the types of tests that will be conducted at each additional site.
07
Attach any required documentation to support your application, such as certificates or licenses.
08
Review the completed application for accuracy and completeness.
09
Sign and date the application at the designated area.
10
Submit the application via the specified method (mail, fax, or electronic submission) to the appropriate authority.
Who needs APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B?
01
Clinical laboratories that wish to expand their services by adding additional testing sites.
02
Laboratories that have opened new locations and need to register these sites for compliance.
03
Entities seeking to ensure that all testing locations meet regulatory requirements.
Fill
form
: Try Risk Free






People Also Ask about
What federal regulatory standards apply to all clinical laboratory testing performed on humans in the United States?
The Clinical Laboratory Improvement Amendments of 1988 (CLIA) regulations apply to all U.S. facilities or sites that test human specimens for health or disease assessment.
Which of these clinical laboratories would need a California clinical laboratory license?
Labs doing moderate or high complexity tests (also called "non-waived tests") must be licensed and inspected every 2 years. Labs doing waived or PPMP tests must be registered with the state and are not routinely inspected.
How many non-waived licensed labs can lab directors direct in California?
DOES THE MAXIMUM LIMIT OF DIRECTING 5 LABORATORIES APPLY IF SOME OF THE LABORATORIES FOR WHICH I AM THE DIRECTOR ONLY PERFORM WAIVED TESTS? No, the maximum limit of directing 5 laboratories (laboratories in this case means the number of certificates) is only applicable for laboratories performing nonwaived tests.
What is lab 144a?
Get the up-to-date lab 144a 2025 now. Get Form. 4.8 out of 5. 36 votes. The document is an application form for obtaining an initial clinical laboratory license from the California Department of Public Health.
What is the difference between a CLIA and a FDA?
Through the Clinical Laboratory Improvement Amendments (CLIA) Program, CMS regulates all lab testing (with some specific exceptions and state exemptions) done on humans in the U.S. to ensure accurate, reliable, and timely patient test results.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B?
APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B is a form used by clinical laboratories to request the addition of new testing sites to their existing operational framework, ensuring compliance with regulatory standards.
Who is required to file APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B?
Clinical laboratories that wish to expand their testing capabilities by adding new locations must file APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B.
How to fill out APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B?
To fill out the APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B, the applicant must provide detailed information about the new testing site, including its address, type of tests conducted, and the laboratory's operational details, ensuring all required fields are completed accurately.
What is the purpose of APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B?
The purpose of APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B is to formalize the process through which clinical laboratories seek approval to operate additional testing locations while adhering to state and federal regulations.
What information must be reported on APPLICATION FOR ADDITIONAL CLINICAL LABORATORY TESTING SITES--FORM B?
The information that must be reported includes the name and address of the proposed testing site, the types of tests to be offered, the management structure of the new site, as well as compliance with laboratory standards and regulatory requirements.
Fill out your application for additional clinical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Application For Additional Clinical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















