Get the free Drug-Induced Side Effects Report - optometry ohio
Show details
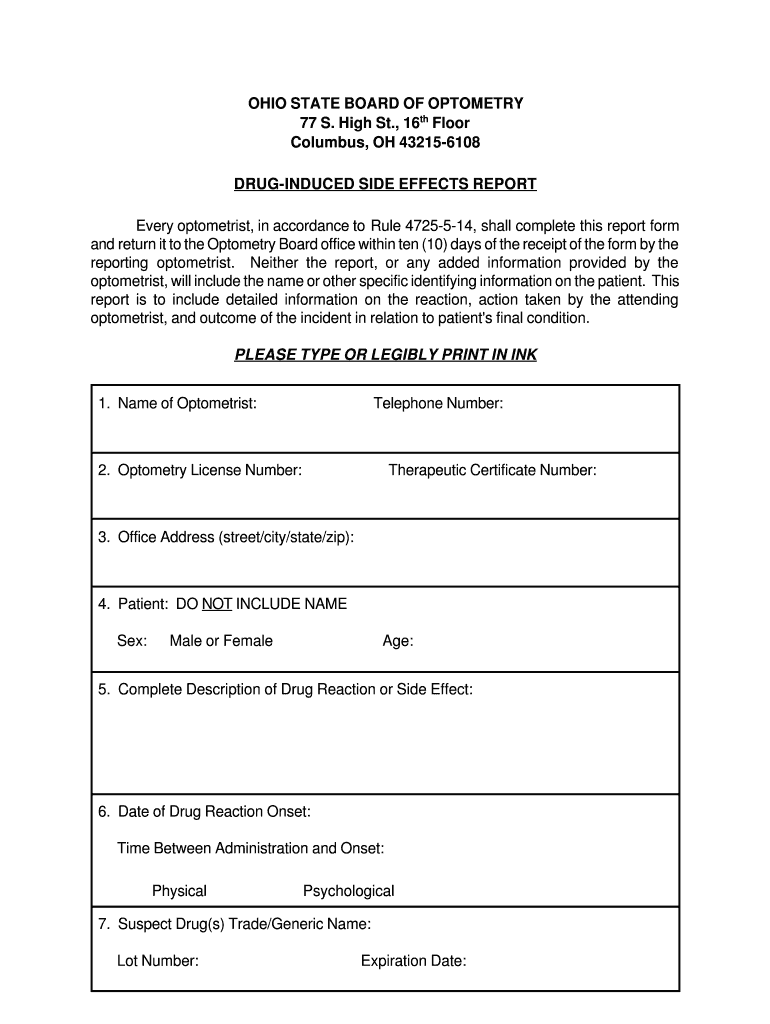
This form is to be completed by optometrists for reporting drug-induced side effects, including detailed information on the reaction, action taken, and the outcome for the patient, in compliance with
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign drug-induced side effects report

Edit your drug-induced side effects report form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your drug-induced side effects report form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing drug-induced side effects report online
Follow the steps down below to use a professional PDF editor:
1
Check your account. It's time to start your free trial.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit drug-induced side effects report. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out drug-induced side effects report

How to fill out Drug-Induced Side Effects Report
01
Begin by writing the date and time of the report.
02
Fill in the name of the patient experiencing side effects.
03
Provide the patient's age and gender.
04
List the medication that the patient is taking, including dosage and administration route.
05
Describe the side effects observed, including onset time and duration.
06
Include any previous medical history relevant to the side effects.
07
Mention any other medications the patient is currently taking.
08
Provide details on any interventions taken and the outcomes.
09
Sign and date the report for validation.
Who needs Drug-Induced Side Effects Report?
01
Healthcare professionals prescribing medication.
02
Pharmacists monitoring patient health.
03
Clinical researchers studying drug effects.
04
Regulatory authorities reviewing drug safety.
05
Patients experiencing side effects for better management.
Fill
form
: Try Risk Free






People Also Ask about
How to report adverse drug effects?
Where to report? Duly filled in Suspected Adverse Drug Reaction Reporting Form can be sent to the nearest Adverse Drug Reaction Monitoring Centre (AMC) or directly to the National Coordination Centre (NCC) for PvPI.
Who to call about medication side effects?
Call your doctor right away if you have any problems with your medicines or if you are worried that the medicine might be doing more harm than good. Your health care provider may be able to prescribe a different medicine or help you deal with side effects in other ways.
How do I report side effects of drugs?
If you need information or if you have questions or comments about a medical product, please call the FDA's toll-free information line, 1-888-INFO-FDA (1-888-463-6332) Press 2 to report into MedWatch or for instructions.
How do you report a drug side effect?
If you need information or if you have questions or comments about a medical product, please call the FDA's toll-free information line, 1-888-INFO-FDA (1-888-463-6332) Press 2 to report into MedWatch or for instructions.
How do I report an ADR?
ADRs can be also reported via PvPI helpline number (18001803024) on weekdays from 9:00 am to 5:30 pm. [3] The mobile Android application for ADR reporting has also been made available to the public.
What is drug induced side effects?
Side effects, also known as adverse reactions, are unwanted undesirable effects that are possibly related to a drug. Side effects can vary from minor problems like a runny nose to life-threatening events, such as a heart attack or liver damage.
Do adverse effects need to be reported?
All suspected side effects should be reported, especially those that are: unexpected, regardless of their severity (i.e. not consistent with product information or labelling);
Is adverse event reporting mandatory?
The Medical Device Reporting (MDR) regulation (21 CFR Part 803) contains mandatory requirements for manufacturers, importers, and device user facilities to report certain device-related adverse events and product problems to the FDA.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Drug-Induced Side Effects Report?
A Drug-Induced Side Effects Report is a document used to record and report any side effects or adverse reactions experienced by individuals as a result of taking a specific medication.
Who is required to file Drug-Induced Side Effects Report?
Healthcare professionals, including doctors, nurses, and pharmacists, as well as patients and caregivers, are required to file a Drug-Induced Side Effects Report when they observe or experience adverse effects from a medication.
How to fill out Drug-Induced Side Effects Report?
To fill out a Drug-Induced Side Effects Report, individuals must provide detailed information about the patient, the medication involved, the nature of the side effects, the time frame in which they occurred, and any other relevant medical history or observations.
What is the purpose of Drug-Induced Side Effects Report?
The purpose of the Drug-Induced Side Effects Report is to gather data on adverse reactions to medications, which can help improve drug safety, inform regulatory bodies, and contribute to better patient care and treatment options.
What information must be reported on Drug-Induced Side Effects Report?
Information that must be reported includes patient demographics, details of the medication (name, dosage, route), description of the side effects experienced, duration of the side effects, any relevant medical history, and actions taken in response to the side effects.
Fill out your drug-induced side effects report online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Drug-Induced Side Effects Report is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















