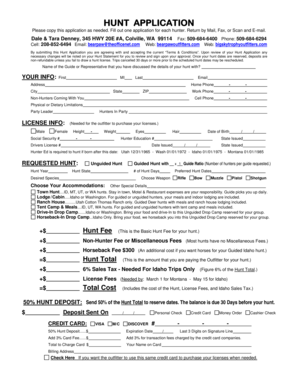
Get the free Clinical Research Associate Certificate Program - dlt ri
Show details
This document outlines the Clinical Research Associate Certificate Program offered by Bryant University Executive Development Center, including course content, costs, contact information, and eligibility
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical research associate certificate
Edit your clinical research associate certificate form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical research associate certificate form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical research associate certificate online
To use the professional PDF editor, follow these steps:
1
Check your account. It's time to start your free trial.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit clinical research associate certificate. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical research associate certificate
How to fill out Clinical Research Associate Certificate Program
01
Research accredited institutions offering the Clinical Research Associate Certificate Program.
02
Review the program outline and prerequisites to ensure eligibility.
03
Gather all necessary documents, such as transcripts and a resume.
04
Complete the online application form or contact the institution for a paper application.
05
Submit payment for the application fee, if applicable.
06
Enroll in the required courses as specified by the program.
07
Attend all classes and complete any mandatory practical training.
08
Pass all assessments and fulfill any internship or project requirements.
09
Apply for graduation or certification upon completion of the program.
Who needs Clinical Research Associate Certificate Program?
01
Individuals looking to start a career in clinical research.
02
Current healthcare professionals seeking to expand their expertise.
03
Students studying life sciences or related fields.
04
Pharmaceutical company employees aiming for career advancement.
05
Anyone interested in understanding the regulatory aspects of clinical trials.
Fill
form
: Try Risk Free






People Also Ask about
Is a certificate in clinical research worth it?
The Benefits of Earning a CRA Certification ing to the Association of Clinical Research Professionals (ACRP), certified professionals say that the achievement of their certification has resulted in better employment options, increased job responsibilities, and expanded advancement opportunities.
How much does CRA training cost?
$495.00. ($1200 Value) 288 In-Depth Advanced CRA Training Modules + ACRAC Study Guide + Certification Exam Backed by CPD + CME Credits. Lifetime Access to Unmatched Curriculum.
Is CRA certification worth it?
The Benefits of Earning a CRA Certification ing to the Association of Clinical Research Professionals (ACRP), certified professionals say that the achievement of their certification has resulted in better employment options, increased job responsibilities, and expanded advancement opportunities.
How to become a certified CRA?
Certification and Education Requirements Most CRA positions require a bachelor's degree in life sciences or related fields and a healthy amount of clinical study experience. Several universities offer graduate certificates or master's degrees in clinical research or clinical trial management.
How to get a CRA license?
You can choose from CRA certifications offered by two different organizations. The ACRP offers the Certified Clinical Research Associate credential. To earn this certification, you must have one of the following: A bachelor's degree and at least 3,000 hours of experience as a CRA.
How much does it cost to get CRA certified?
$495.00. ($1200 Value) 288 In-Depth Advanced CRA Training Modules + ACRAC Study Guide + Certification Exam Backed by CPD + CME Credits. Lifetime Access to Unmatched Curriculum.
Which certification is best for clinical research?
CCRC® (Certified Clinical Research Coordinator) is a credential formally recognizing clinical research professionals with experience coordinating and facilitating clinical trial activities in adherence to GCP, under the direction of a principal investigator.
How much does the CRA exam cost?
Applications and fees are as follows: AHRA Members: The exam fee is $325, plus a $100 application fee to verify eligibility. The total of $425 (plus late fee when applicable) must accompany the application. All Other Applicants: The exam fee is $500, plus a $100 application fee to verify eligibility.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Clinical Research Associate Certificate Program?
The Clinical Research Associate Certificate Program is an educational course designed to provide individuals with the necessary knowledge and skills to work as clinical research associates (CRAs) in the pharmaceutical and clinical research industry.
Who is required to file Clinical Research Associate Certificate Program?
Individuals who wish to pursue a career as clinical research associates or those looking to enhance their qualifications in clinical research are typically required to complete the Clinical Research Associate Certificate Program.
How to fill out Clinical Research Associate Certificate Program?
To fill out the Clinical Research Associate Certificate Program, candidates need to complete the application form provided by the educational institution offering the program, include their personal information, educational background, and any relevant work experience.
What is the purpose of Clinical Research Associate Certificate Program?
The purpose of the Clinical Research Associate Certificate Program is to train individuals in the fundamental principles of clinical research, regulatory requirements, and the role of a CRA, preparing them for employment in the field.
What information must be reported on Clinical Research Associate Certificate Program?
The information that must be reported includes candidate's personal details, educational qualifications, relevant work experience, contact information, and any supporting documents required by the program.
Fill out your clinical research associate certificate online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Research Associate Certificate is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.