Get the free Medical Visors MV100 510(k) Submission - accessdata fda
Show details
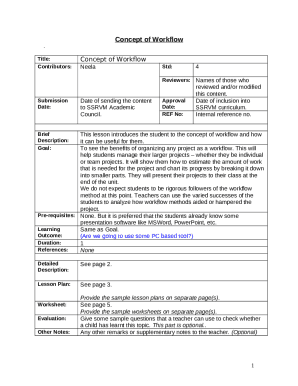
This document provides a summary of safety and effectiveness information for the Medical Visors MV100 device intended for use during MRI procedures to improve patient comfort.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign medical visors mv100 510k

Edit your medical visors mv100 510k form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your medical visors mv100 510k form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing medical visors mv100 510k online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Sign into your account. It's time to start your free trial.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit medical visors mv100 510k. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
With pdfFiller, it's always easy to deal with documents. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out medical visors mv100 510k

How to fill out Medical Visors MV100 510(k) Submission
01
Gather all necessary information about the Medical Visor MV100.
02
Review the FDA guidelines for 510(k) submissions to understand the requirements.
03
Complete the 510(k) submission form, including the product description and intended use.
04
Provide performance testing data, including safety and efficacy information.
05
Include labeling and packaging details for the Medical Visor MV100.
06
Prepare any required clinical data or summaries if applicable.
07
Compile a list of predicate devices for comparison.
08
Review and finalize the submission package for completeness.
09
Submit the 510(k) application electronically via the FDA's submission portal.
10
Monitor the status of your submission and respond to any FDA inquiries.
Who needs Medical Visors MV100 510(k) Submission?
01
Manufacturers of the Medical Visors MV100 seeking FDA clearance.
02
Healthcare providers looking to market or distribute the Medical Visor MV100.
03
Distributors wanting authorization to sell Medical Visors MV100 products.
Fill
form
: Try Risk Free






People Also Ask about
Can you see 510k submissions?
The FDA maintains a database of premarket notification 510(k) submissions on its official website, enabling users to search for existing submissions using criteria such as the 510(k) number, applicant, device name or FDA product code.
How much does a 510k submission cost?
How Much Does a FDA 510k Approval Cost? The vast majority of our FDA 510K clients generally spend in the range of $20,000-$30,000 to have their product or device prepared and reviewed before the actual FDA 510k submission process.
How much does it cost to submit a 510k?
How Much Does a FDA 510k Approval Cost? The vast majority of our FDA 510K clients generally spend in the range of $20,000-$30,000 to have their product or device prepared and reviewed before the actual FDA 510k submission process.
What does it mean to be 510k cleared?
It's important to note that clearance under the 510(k) pathway does not indicate that the FDA has approved the device but rather that the FDA has determined the device is equivalent to a similar device already on the market.
How to find 510k submissions?
Users can search the FDA 510(k) database by entering the name of a specific medical device, the name of the applicant who filed the 510(k) premarket notification paperwork for the device, or by entering the specific 510(k) number or product code associated with a given device.
Can you see FDA submissions?
The FDA secures the information about each submission's progress to ensure only the official correspondent or designated delegates* for that submission can view the status.
How much is the FDA entry fee?
Annual Establishment Registration Fee: $9,280 All establishments must pay the establishment registration fee. There are no waivers or reductions for small establishments, businesses, or groups in FY 2025.
What is 510k clearance for medical devices?
The 510k clearance process is the most common pre-market pathway used by medical device companies to legally sell products in the US, as data from the FDA shows that: 35% of medical devices are in Class I, the lowest risk class, as they present minimal potential for harm to the end user.
How much does it cost to get a product FDA approved?
Consulting Fees for US agent services (typically an annual fee ranging from $250-$1,500) Any additional consulting fees if the FDA contacts your agent. Who is responsible for payment of FDA User Fees ($9,280 for FY 2025 FDA User Fee)
Is 510k the same as FDA approval?
The FDA 510k submission requirements include extensive technical documentation such as proposed labeling, sterilization and shelf life data, biocompatibility research, animal, clinical and bench performance testing and other technical evidence needed to establish substantial equivalence and receive FDA 510k clearance.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Medical Visors MV100 510(k) Submission?
The Medical Visors MV100 510(k) Submission is a premarket submission made to the FDA to demonstrate that a medical device, in this case the MV100 visor, is safe and effective for its intended use. It allows manufacturers to seek clearance to market and sell the device in the United States.
Who is required to file Medical Visors MV100 510(k) Submission?
Manufacturers and distributors of the Medical Visors MV100 who wish to market the device in the United States are required to file a 510(k) submission with the FDA. This includes companies that produce devices that are not exempt from 510(k) requirements.
How to fill out Medical Visors MV100 510(k) Submission?
To fill out a Medical Visors MV100 510(k) Submission, manufacturers must complete the FDA's 510(k) form, provide a description of the device, details on its intended use, and any applicable data demonstrating its safety and efficacy. This includes information on testing, labeling, and manufacturing processes.
What is the purpose of Medical Visors MV100 510(k) Submission?
The purpose of the Medical Visors MV100 510(k) Submission is to obtain FDA clearance for marketing the medical visor by demonstrating that it is substantially equivalent to a legally marketed device, thereby ensuring its safety and effectiveness for users.
What information must be reported on Medical Visors MV100 510(k) Submission?
The information that must be reported in the Medical Visors MV100 510(k) Submission includes device identification, description and labeling, intended use, substantial equivalence comparison to predicate devices, performance data, and manufacturing information, including quality control processes.
Fill out your medical visors mv100 510k online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Medical Visors mv100 510k is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.