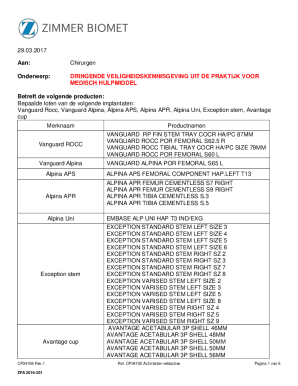
Get the free SectionS5 510(k) Summary - accessdata fda
Show details
SectionS5: 510(k) Summary K (W766 p 1oPRZ2Applied Cardiac Systems Abbreviated Submission AUG 2 42011 Section 5: 510(k) Summary Submitter: Company: Address: Contact: Phone: Fax: Email: Date Prepared:
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign sections5 510k summary

Edit your sections5 510k summary form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your sections5 510k summary form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit sections5 510k summary online
To use our professional PDF editor, follow these steps:
1
Log in to account. Start Free Trial and register a profile if you don't have one yet.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit sections5 510k summary. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out sections5 510k summary

How to fill out sections5 510k summary?
01
Start by reviewing the requirements and guidelines provided by the regulatory authority, such as the Food and Drug Administration (FDA) in the United States.
02
Follow the specified format for the summary, ensuring that all required sections are included. These sections may vary depending on the specific regulations and guidelines applicable to your product.
03
Begin by providing a brief introduction to the medical device, describing its intended use, and highlighting its features.
04
Include a detailed description of the device's technological characteristics, construction materials, and manufacturing processes. This section should also address any clinical or technical performance data available, as well as any design validations or verifications conducted.
05
Clearly outline the intended indications for use, specifying the patient population and the medical conditions or diseases that the device is intended to diagnose, treat, prevent, or mitigate.
06
Discuss any previous clearances or approvals obtained for this device or similar devices. This may include referencing any relevant predicate devices and demonstrating substantial equivalence if required.
07
Provide a comprehensive summary and analysis of the clinical data supporting the safety and effectiveness of the device. This may include clinical studies, literature reviews, post-market surveillance data, or expert opinions.
08
Include a detailed explanation of any potential risks associated with the device and the mitigation measures in place to address them. Additionally, discuss any known contraindications, warnings, or precautions that users should be aware of.
09
Document any labeling claims, including indications for use, contraindications, warnings, precautions, and any special considerations for use. This section should also cover the instructions for use, including any necessary training requirements.
Who needs sections5 510k summary?
01
Manufacturers of medical devices seeking clearance or approval from regulatory authorities, such as the FDA in the United States, need to submit a sections5 510k summary. This summary provides detailed information about the device, its intended use, and supporting data to demonstrate its safety and effectiveness.
02
Regulatory authorities, such as the FDA, require the sections5 510k summary to assess the device's compliance with applicable regulations and guidelines. This summary helps the authorities evaluate the device's risk classification, substantial equivalence to predicate devices, and overall safety and efficacy.
03
Healthcare professionals, including physicians, nurses, and other medical personnel, may review the sections5 510k summary to gain a better understanding of the device's intended use, indications, contraindications, and potential risks. This information allows them to make informed decisions regarding the use of the device in patient care.
04
Patients and consumer advocacy groups may also benefit from reviewing sections5 510k summaries, as they provide insights into the safety and effectiveness of medical devices. Access to this information can empower patients to make informed decisions about their healthcare and raise concerns about specific devices if necessary.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is sections5 510k summary?
The 510k summary is a summary of the premarket notification process for medical devices regulated by the Food and Drug Administration (FDA) under section 510(k) of the Federal Food, Drug, and Cosmetic Act.
Who is required to file sections5 510k summary?
Manufacturers or distributors of medical devices intending to introduce a new device to the market are required to file a 510k summary.
How to fill out sections5 510k summary?
To fill out a 510k summary, the manufacturer or distributor must provide detailed information about the device, its intended use, its components, its performance data, and any other relevant information required by the FDA.
What is the purpose of sections5 510k summary?
The purpose of the 510k summary is to demonstrate that the new medical device is substantially equivalent to a legally marketed device and does not pose a significant risk to public health.
What information must be reported on sections5 510k summary?
The 510k summary must include information such as the device's intended use, its design, its materials, its performance data, any clinical studies conducted, and a comparison to similar devices already on the market.
How do I complete sections5 510k summary online?
pdfFiller has made filling out and eSigning sections5 510k summary easy. The solution is equipped with a set of features that enable you to edit and rearrange PDF content, add fillable fields, and eSign the document. Start a free trial to explore all the capabilities of pdfFiller, the ultimate document editing solution.
How do I make edits in sections5 510k summary without leaving Chrome?
Install the pdfFiller Google Chrome Extension in your web browser to begin editing sections5 510k summary and other documents right from a Google search page. When you examine your documents in Chrome, you may make changes to them. With pdfFiller, you can create fillable documents and update existing PDFs from any internet-connected device.
How can I edit sections5 510k summary on a smartphone?
The pdfFiller mobile applications for iOS and Android are the easiest way to edit documents on the go. You may get them from the Apple Store and Google Play. More info about the applications here. Install and log in to edit sections5 510k summary.
Fill out your sections5 510k summary online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

sections5 510k Summary is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.