Get the free Promos Modular Shoulder System 510(k) Premarket Notification - accessdata fda
Show details
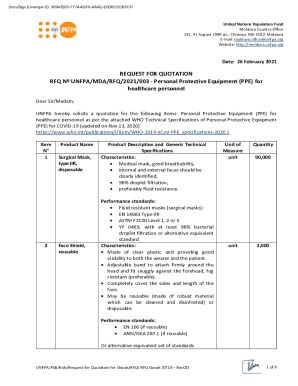
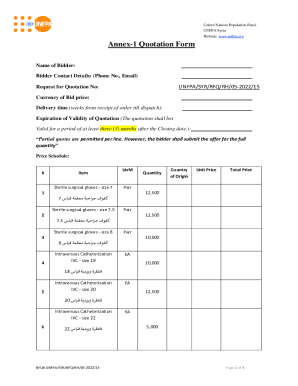
This document is a 510(k) premarket notification submitted to request clearance for the modified inclination sets of the Promos Modular Shoulder System, detailing design specifications, intended use,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign promos modular shoulder system

Edit your promos modular shoulder system form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your promos modular shoulder system form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit promos modular shoulder system online
To use the services of a skilled PDF editor, follow these steps below:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit promos modular shoulder system. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
Dealing with documents is simple using pdfFiller. Try it now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out promos modular shoulder system

How to fill out Promos Modular Shoulder System 510(k) Premarket Notification
01
Gather all necessary documentation, including device descriptions, intended use, and labeling.
02
Prepare a summary of safety and effectiveness information.
03
Identify the predicate device to which you are claiming equivalence.
04
Complete the 510(k) application form, including administrative information.
05
Submit detailed performance testing data and results.
06
Include biocompatibility test results if applicable.
07
Provide a sterilization method and validation if the device is intended to be sterilized.
08
Submit the 510(k) application to the FDA.
09
Wait for FDA feedback and be prepared to provide additional information or clarification.
Who needs Promos Modular Shoulder System 510(k) Premarket Notification?
01
Medical device manufacturers seeking to market the Promos Modular Shoulder System in the United States.
02
Healthcare providers and practitioners intending to use or recommend the device after its approval.
Fill
form
: Try Risk Free






People Also Ask about
How long does it take for the 510 K determination in the case of traditional premarket notification?
Information from the Design Controls process is crucial. The FDA usually takes 30-90 days to process 510(k) submissions—however, the entire premarket notification process can take much longer. If accepted, the submission is listed in the database. Generally, Class II medical devices require a 510k submission.
What is 510k premarket notification?
A 510(k) Premarket Notification is required for low to moderate risk medical devices classified as either Class I or Class II and not exempt from 510(k). Companies are unable to market their products to health care providers, patients and other consumers until they are given 510(k) clearance by the FDA.
What is a 510 K premarket notification?
Information from the Design Controls process is crucial. The FDA usually takes 30-90 days to process 510(k) submissions—however, the entire premarket notification process can take much longer. If accepted, the submission is listed in the database. Generally, Class II medical devices require a 510k submission.
What is an exemption from the 510k premarket notification requirements?
Most class I and class II devices are exempt from 510(k) requirements. Devices that may be 510(k) exempt include: Preamendment devices (devices legally marketed in the US prior to May 28, 1976) Class I devices specifically exempted by FDA or classified as class I under section 513 (with certain exceptions)
What is pre-market notification?
Premarket notification is the process through which these new devices claim substantial equivalency to a preexisting device and are automatically classified into the same class as that predicate device.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Promos Modular Shoulder System 510(k) Premarket Notification?
The Promos Modular Shoulder System 510(k) Premarket Notification is a submission to the FDA demonstrating that the device is safe and effective for its intended use, and is substantially equivalent to a legally marketed device.
Who is required to file Promos Modular Shoulder System 510(k) Premarket Notification?
Manufacturers and importers of the Promos Modular Shoulder System who intend to market the device in the U.S. are required to file the 510(k) Premarket Notification.
How to fill out Promos Modular Shoulder System 510(k) Premarket Notification?
To fill out the Promos Modular Shoulder System 510(k) Premarket Notification, submitters must complete the FDA Form 3514, include the device description, indications for use, labeling, and any necessary performance testing data.
What is the purpose of Promos Modular Shoulder System 510(k) Premarket Notification?
The purpose of the Promos Modular Shoulder System 510(k) Premarket Notification is to obtain FDA clearance for marketing the device in the U.S. by showing that it is safe and effective.
What information must be reported on Promos Modular Shoulder System 510(k) Premarket Notification?
Information that must be reported includes the device's name, intended use, description, design specifications, labeling, and any data from biocompatibility and performance tests.
Fill out your promos modular shoulder system online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Promos Modular Shoulder System is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.