Get the free 510(k) Summary for Glow by EndyMed - accessdata fda
Show details
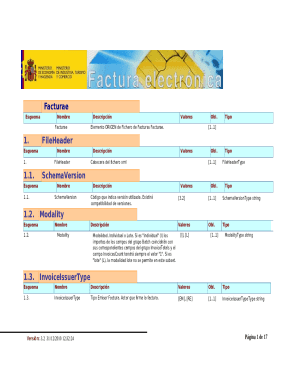
This document provides a summary of the 510(k) notification process for the Glow by EndyMed device, detailing its intended use, device description, predicate devices, and compliance with performance
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign 510k summary for glow

Edit your 510k summary for glow form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your 510k summary for glow form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing 510k summary for glow online
To use the services of a skilled PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit 510k summary for glow. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out 510k summary for glow

How to fill out 510(k) Summary for Glow by EndyMed
01
Gather the necessary information about the Glow device, including its intended use and technological characteristics.
02
Review the requirements set by the FDA for a 510(k) summary to ensure compliance.
03
Identify the predicate device that the Glow device is being compared to in the 510(k) submission.
04
Clearly describe the device and its components, including materials and manufacturing processes.
05
Detail the performance testing and data supporting the device's safety and effectiveness.
06
Summarize the risk assessment and mitigation measures taken during the device's development.
07
Provide any labeling information needed for users and practitioners.
08
Submit the completed 510(k) summary along with any required documentation to the FDA.
Who needs 510(k) Summary for Glow by EndyMed?
01
Manufacturers of the Glow by EndyMed who wish to market their medical device in the United States.
02
Healthcare professionals seeking to understand the regulatory status and safety data of the Glow device.
03
Research and development teams involved in the device's design and compliance with FDA regulations.
Fill
form
: Try Risk Free






People Also Ask about
Is Endymed FDA approved?
The ENDYMED PRO, powered by unique, FDA cleared 3DEEP RF technology, offers innovative and effective aesthetic solutions for non-surgical lifting, tightening, contouring, skin resurfacing and RF Microneedling for the face, neck and body.
How much does Endymed skin tightening cost?
($1500 per treatment. Immediately after your tightening and contouring treatment, you will notice contraction of the skin in the area treated. Results will continue to improve over the next few months as your collagen and elastin production increases.
Does endymed actually work?
Clinical Study Verdict: 86% - 100% Achieving 'Good' to 'Excellent' Results. Endymed RF treatments have been the subject of several clinical trials, with results varying from 86% to 100% of patients achieving “Good to Excellent” results.
What is the FDA approved skin tightening treatment?
Ultherapy® is an FDA-approved treatment that uses a high-intensity, focused ultrasound to reach the deep structures of the face to lift and tighten.
What are the side effects of Endymed skin tightening?
Common side effects include mild redness, swelling, and sensitivity in the treated area, which generally subside within a few days. It's crucial to follow post-treatment care instructions provided by the skincare professional to ensure proper healing and optimal results.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is 510(k) Summary for Glow by EndyMed?
The 510(k) Summary for Glow by EndyMed is a premarket submission to the FDA demonstrating that the Glow device is safe and effective, and is substantially equivalent to a legally marketed device.
Who is required to file 510(k) Summary for Glow by EndyMed?
The manufacturer or company that produces the Glow by EndyMed device is required to file the 510(k) Summary with the FDA.
How to fill out 510(k) Summary for Glow by EndyMed?
To fill out the 510(k) Summary for Glow by EndyMed, the manufacturer must compile information including the device description, indications for use, technological characteristics, and data demonstrating substantial equivalence.
What is the purpose of 510(k) Summary for Glow by EndyMed?
The purpose of the 510(k) Summary for Glow by EndyMed is to inform the FDA and the public about the device's safety and effectiveness and to obtain clearance to market the device.
What information must be reported on 510(k) Summary for Glow by EndyMed?
The 510(k) Summary must include device identification, a statement of intended use, performance testing data, labeling information, and comparisons with the predicate device.
Fill out your 510k summary for glow online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

510k Summary For Glow is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.