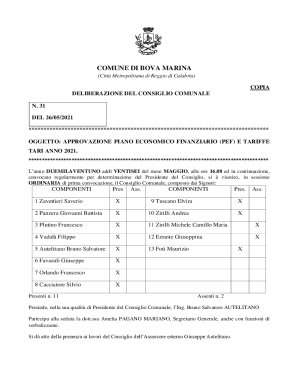
Get the free Risk Evaluation and Mitigation Strategy (REMS) Memorandum - accessdata fda
Show details
This document evaluates the risk factors associated with the SUPREP Bowel Prep Kit and outlines a Risk Evaluation and Mitigation Strategy (REMS) to ensure patient safety regarding the medication's
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign risk evaluation and mitigation

Edit your risk evaluation and mitigation form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your risk evaluation and mitigation form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing risk evaluation and mitigation online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit risk evaluation and mitigation. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out risk evaluation and mitigation

How to fill out Risk Evaluation and Mitigation Strategy (REMS) Memorandum
01
Obtain the Risk Evaluation and Mitigation Strategy (REMS) template from the relevant regulatory authority.
02
Review the requirements for the specific drug or product to ensure compliance.
03
Fill out the identification section with the name of the drug, indication, and sponsor information.
04
Conduct a thorough risk assessment identifying potential risks associated with the drug.
05
Develop strategies to mitigate identified risks, detailing specific actions to be taken.
06
Outline the communication plan for informing healthcare providers and patients about the REMS.
07
Establish a monitoring and assessment plan to evaluate the effectiveness of the REMS.
08
Complete any required data collection and submission schedules.
09
Submit the finished REMS memorandum to the regulatory authority for review.
Who needs Risk Evaluation and Mitigation Strategy (REMS) Memorandum?
01
Pharmaceutical companies developing drugs with known risks.
02
Healthcare providers who prescribe medications that require monitoring.
03
Patients prescribed medications that have a REMS in place.
04
Regulatory authorities overseeing drug safety and efficacy.
Fill
form
: Try Risk Free






People Also Ask about
What is the FDA's risk evaluation and mitigation strategy?
Risk mitigation is the practice of reducing the impact of potential risks by developing a plan to manage, eliminate, or limit setbacks as much as possible. After management creates and carries out the plan, they'll monitor progress and assess whether or not they need to modify any actions.
What is a risk evaluation and mitigation strategy?
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks.
What best describes the risk evaluation and mitigation strategy?
A Risk Evaluation and Mitigation Strategy (REMS) is a formal plan required by the FDA for certain “riskier” drugs to ensure that the benefits of these drugs outweigh their risks.
What is the risk evaluation and mitigation strategy?
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks.
What best describes the risk evaluation and mitigation strategy (REMS)?
A Risk Evaluation and Mitigation Strategy (REMS) is a formal plan required by the FDA for certain “riskier” drugs to ensure that the benefits of these drugs outweigh their risks.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Risk Evaluation and Mitigation Strategy (REMS) Memorandum?
The Risk Evaluation and Mitigation Strategy (REMS) Memorandum is a formal document that outlines a specific strategy designed to manage the risks associated with a particular medication. REMS is required by the FDA to ensure that the benefits of a drug outweigh its risks by implementing specific requirements for its safe use.
Who is required to file Risk Evaluation and Mitigation Strategy (REMS) Memorandum?
Pharmaceutical manufacturers are required to file a Risk Evaluation and Mitigation Strategy (REMS) Memorandum with the FDA when they seek approval for a new drug or when new safety concerns arise regarding an already marketed drug. This filing is part of the regulatory process to ensure patient safety.
How to fill out Risk Evaluation and Mitigation Strategy (REMS) Memorandum?
To fill out a REMS Memorandum, the applicant must provide detailed information about the medication, including its risks and benefits, the proposed REMS elements such as communication plans, and how these elements will be implemented and monitored. Guidance documents provided by the FDA should be followed closely to ensure compliance.
What is the purpose of Risk Evaluation and Mitigation Strategy (REMS) Memorandum?
The purpose of the REMS Memorandum is to ensure that necessary strategies are put in place to mitigate the risks associated with certain medications, thereby enhancing patient safety, promoting responsible prescribing, and ensuring that patients have access to effective treatments while minimizing potential harm.
What information must be reported on Risk Evaluation and Mitigation Strategy (REMS) Memorandum?
The REMS Memorandum must report information such as the drug's brand and generic names, the elements to assure safe use (ETASU) that are required, the communication plan for healthcare providers and patients, and the evaluation process to assess the effectiveness of the REMS over time.
Fill out your risk evaluation and mitigation online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Risk Evaluation And Mitigation is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.